Drug-induced hypersensitivity syndrome is a toxicoderma with systemic involvement. Suspicion of this disorder obliges rapid withdrawal of the suspected drug, which may have been introduced up to 3 months earlier. Screening for human herpesvirus (HHV) 6 reactivation is important both for its diagnostic value and for its association with a poor prognosis. Reactivation of this virus is not a contraindication for systemic corticosteroid treatment, which should be tapered slowly in order to avoid recurrence. The possible appearance of antiphospholipid antibodies must also be considered in those cases associated with thrombocytopenia, altered hemostasis, or thrombotic events. Autoimmune disorders may also develop as a sequela of the condition. Medium-to-long-term follow-up is required even after complete resolution of the condition. We describe a new case of sulfasalazine-induced hypersensitivity syndrome associated with HHV-6 reactivation and the induction of anticardiolipin and anti-thyroid peroxidase antibodies.
El síndrome de hipersensibilidad inducido por fármacos (DIHS) se engloba dentro de los cuadros toxicodérmicos con afectación sistémica, cuya sospecha obliga a la retirada lo más precoz posible del fármaco que consideremos pueda estar implicado, que puede haber sido introducido hasta tres meses antes.
Es importante el despistaje de la reactivación del virus herpes humano (VHH) 6, tanto por su valor diagnóstico como por el pronóstico de gravedad que supone. Por otro lado, esta reactivación no contraindica que el tratamiento pueda llevarse a cabo con corticoides sistémicos, que además, deberán retirarse lentamente para evitar recaídas. Debería pensarse asimismo en la posibilidad de la presencia de anticuerpos antifofolipídicos en los casos en que apareciera trombopenia, alteración en la hemostasia o cuadros trombóticos asociados, como también tener en cuenta el desarrollo de procesos autoinmunes como secuela del cuadro, que requiere de monitorización y seguimiento a medio-largo plazo, a pesar de la resolución completa del cuadro.
Describimos un nuevo caso donde se muestra la reactivación del VHH-6, la activación de anticuerpos anticardiolipina y la inducción de anticuerpos anti-tiroperoxidasa (anti-TPO).
Drug-induced hypersensitivity syndrome (DIHS), also known as drug reaction with eosinophilia and systemic symptoms1 or drug-induced delayed multiorgan hypersensitivity syndrome,2 is a drug hypersensitivity disorder and must be suspected in any patient who develops fever, a rash, hepatitis, lymphadenopathy, and leukocytosis (with or without eosinophilia) between 2 weeks and 3 months after starting treatment with certain drugs (Table 2).
Drugs Reported to Cause Drug-Induced Hypersensitivity Syndrome.
| Anticonvulsants |
| Carbamazepine, phenytoin, phenobarbital, lamotrigine, zonisamide |
| Allopurinol |
| Dapsone |
| Antimicrobials |
| Minocycline and doxycycline, vancomycin, trimethoprim-sulfamethoxazole, beta-lactams, metronidazole, carbapenem, abacavir, and nevirapine |
| Salazosulfapyridine |
| Spironolactone |
| Carbimazole |
| Fluindione |
| Nonsteroidal anti-inflammatory drugs |
| β-blockers |
| Angiotensin-converting enzyme inhibitors |
| Thalidomide |
| Ranitidine |
| Methyldopa |
| Calcium channel blockers |
| Quinine |
| Epoetin alfa |
| Bupropion |
| Biological therapy: |
| Imatinib, efalizumab |
The involvement of human herpesvirus (HHV) 6has been reported by a number of study groups and has even been considered a risk factor for severity.3 The relationship between HHV-6 reactivation and the severity of DIHS has led to such reactivation being considered a diagnostic criterion.4
Activation of antiphospholipid antibodies5 and anti-thyroid peroxidase (anti-TPO) antibodies6 in these syndromes has also been reported, although how this may affect the severity of the disorder is unknown. The presence of such antibodies is not used for diagnostic purposes.
We present the case of a patient diagnosed with DIHS in whom we were able to demonstrate HHV-6 reactivation and the presence of antiphospholipid antibodies.
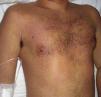
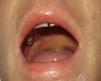
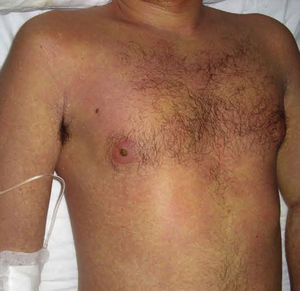
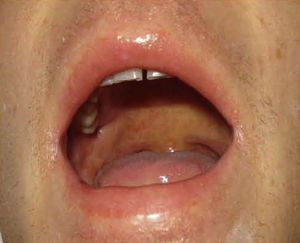
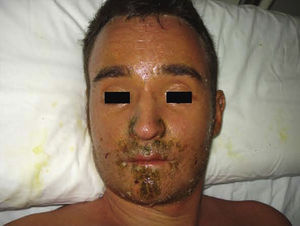
Case DescriptionThe patient, a 27-year-old man, was transferred to our hospital from another center on suspicion of an acute lymphoproliferative syndrome. The only relevant finding in his past history was that he had started treatment with sulfasalazine one month earlier for chondromalacia patellae. At the time of admission he had fever of 40°C, a confluent maculopapular rash with a negative Nikolsky sign (Fig. 1), dyspepsia, and biliary vomiting. He also presented a 1-week history of pharyngoamygdalitis with a whitish exudate, a purpuric rash on the soft palate (Fig. 2), and loss of the lingual papillae for which he had received antibiotic treatment with cefuroxime and paracetamol, with no improvement after a week of therapy. Physical examination revealed hepatosplenomegaly, jaundice (Fig. 3), and distal purpuric edema of the upper and lower limbs, associated with a painful lateral cervical and inguinal lymphadenopathy with mobile lymph nodes measuring 2 to 3cm in diameter. Laboratory test results showed leukocytosis of 34 700/μL with left shift (neutrophils, 14 650/μL; lymphocytes, 12 750/μL); hemoglobin, 12.5g/dL, platelets, 384 x 103/μL; prothrombin time, 20.1 s; prothrombin index, 58%; total proteins, 4.6g/dL; urea, 90mg/dL; creatinine 1.55mg/dL; sodium, 126 mEq/L; potassium, 5.5mEq/L; bilirubin, 10.1mg/dL; aspartate aminotransferase, 628IU/L; alanine aminotransferase, 521IU/L; and lactate dehydrogenase, 2219IU/L. There was no eosinophilia.
Nagayama spots. Purpuric rash on the soft palate and base of the uvula, characteristic of human herpesvirus 6 infection.10
Thyroid function studies revealed a free thyroxine (T4) level of 1.01ng/dL and a thyroid-stimulating hormone (TSH) concentration of 0.066mIU/L. Anti-TPO antibodies were detected at a level of 0.3IU/mL.
There were no abnormalities in cytometry, which suggested that the condition was reactive in origin.
On skin biopsy, there were occasional necrotic keratinocytes in the epidermis with vacuolar degeneration of the basal layer and a predominantly lymphoid inflammatory infiltrate in the superficial dermis, findings consistent with toxicoderma. Computed tomography of neck, thorax, abdomen, and pelvis revealed multiple lymph nodes bilaterally.
Serology (enzyme-linked immunosorbent assay) for Epstein-Barr virus (EBV) (viral capsid antigen [VCA]), herpes simplex virus, cytomegalovirus (CMV), hepatitis A, B, and C viruses, parvovirus B19, measles virus, Toxoplasma, Chlamydia trachomatis, Chlamydia pneumoniae, Chlamydia psittaci, Coxiella burnetti, Legionella, Mycoplasma pneumoniae, and human immunodeficiency virus, was positive for CMV immunoglobulin (Ig) G, EBV (VCA), and HHV-6 IgM and IgG. In addition, IgM anticardiolipin antibodies were later found to be positive.
On the basis of these findings and the past history of treatment with sulfasalazine, DIHS was diagnosed and treatment was started with intravenous methylprednisolone at a dose of 1mg/kg/day. This treatment produced a marked initial improvement, but progressive tapering of the dose and withdrawal of the corticosteroid led to recurrence of the condition. It was therefore decided to recommence therapy at the initial dose and taper more slowly.
Follow-up at 12 weeks found the patient to have marked hair loss on the scalp, but he was otherwise asymptomatic. Anticardiolipin IgG and IgM levels were within the normal ranges. However, thyroid function remained abnormal as follows: free T4, 0.41ng/dL; TSH, 144.952mU/L; and anti-TPO antibody levels of 57.36U/mL.
DiscussionWe present a case of DIHS, an uncommon but severe toxicoderma whose etiology, pathogenesis, and prognosis are related to HHV-6 reactivation.4 In some patients it can lead to the transient induction of anticardiolipin antibodies or an antiphospholipid syndrome.5 The appearance of autoimmune disorders after an asymptomatic period is also relatively common.7
Symptom severity is related to the duration of exposure to the drug after onset of the condition (Table 2).8 Recently, HHV-6 reactivation has also been included as a diagnostic criterion (Table 1)9 and has been linked to more severe disease because, in some series, it was associated with prolongation of the febrile state due to the severity of the hepatitis or renal failure; viral reactivation is a common finding in the cases with a fatal outcome.3,4 In addition, in our case we observed papular erythematous lesions on the soft palate and base of the uvula, known as Nagayama spots (Fig. 2); these are seen in up to 65% of patients with HHV-6 infection. Although many pediatricians use this sign as an early marker of infection, its specificity and predictive value continue to be a subject of discussion.10
Diagnostic Criteria for Drug-Induced Hypersensitivity Syndrome.a
| 1. Erythematous morbilliform rash 3 weeks after starting a new medication |
| 2. Persistence of symptoms at least 2 weeks after withdrawal of the medication |
| 3. Fever >38°C |
| 4. Dysfunction of the liver (alanine aminotransferase > 100 U/L), kidney, or lungs |
| 5. Alterations of white cell populations: leukocytosis >11 000/mm3, atypical lymphocytosis >5%, and/or eosinophilia >1500/mm3 |
| 6. Lymphadenopathies |
| 7. Human herpesvirus 6 reactivation |
Adapted from Shiohara T et al.9
It has also been suggested that HHV-6 may play a possible pathogenic role in the induction of an antiphospholipid syndrome.5,11 In many cases, as in the one we present, the presence of anticardiolipin antibodies is transient and the patient becomes antibody-negative after 2 to 3 months.11 In our patient, despite the absence of signs of thrombosis consistent with antiphospholipid syndrome, anticardiolipin antibodies were detected at the onset of the disorder. We therefore recommend requesting laboratory studies for anticardiolipin antibodies, antinuclear antigen, and extractable nuclear antigen, in addition to markers of hemostasis, to monitor the clinical course of this dermatosis.
It is important to draw attention to the relative frequency with which autoimmune diseases can develop after resolution of the acute disorder; their pathogenesis is associated with suppressor T-cell dysfunction, cytotoxic T-cell activation and proliferation, and the appearance of autoantibodies, especially if preceded by viral reactivation.7 In particular, thyroid function must be monitored for many months after resolution of the condition, as hypothyroidism tends to be asymptomatic initially, but there is early induction of anti-TPO antibodies and a progressive rise in TSH concentrations and fall in free T4 levels, indicative of severe hypothyroidism.6
The treatment of choice, in addition to withdrawal of the culprit drug, consists of systemic corticosteroid therapy. However, DIHS carries a mortality of up to 20%.12 Despite the controversy concerning the use of corticosteroids in the context of a possible viral infection, the cases described in the literature have presented a favorable clinical course after starting this treatment.4,13 It should also be emphasized that the dose of corticosteroid should be tapered slowly in order to avoid potential recurrences14,15; this was done in our case.
In conclusion, when DIHS is suspected, the drug considered to be the cause must be withdrawn as early as possible. It should be noted that the causative drug may have been introduced up to 3 months before the onset of the condition. It is also important to screen for HHV-6 reactivation as not only is this one of the diagnostic criteria, but it also has prognostic value. Furthermore, treatment with systemic corticosteroids rather than being contraindicated is actually the treatment of choice, and it should be withdrawn slowly to prevent recurrence. In patients who develop thrombocytopenia, alterations of hemostasis, or associated thrombotic disorders, the presence of antiphospholipid antibodies should also be investigated. Finally, the possibility of the patient developing autoimmune disease as a sequela of the condition must also be considered; this requires medium-to-long-term monitoring and follow-up even after complete resolution of the syndrome.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Tung Y, et al. Síndrome de hipersensibilidad inducido por sulfasalazina asociado a reactivación de VHH-6 e inducción de síndrome antifosfolípido. Actas Dermosifiliogr. 2011;102:537-540.