Mycosis fungoides (MF) is a cutaneous T-cell lymphoma (CTCL) that typically presents as patches or plaques in sun-protected areas. However, MF frequently exhibits clinical variability, and its atypical presentations can make the diagnosis difficult. Histopathology classically shows an atypical, superficial lymphoid infiltrate with epidermotropism. In addition, molecular assays can demonstrate a dominant T-cell clone in the skin, and flow cytometry can illustrate immunophenotypic abnormalities characteristic of MF. Zosteriform MF is an exceedingly rare variant in which lesions occur in a dermatomal distribution.1–3 We describe the first case of zosteriform MF that responded to antiviral therapy. Interestingly, the patient's MF recurred on four occasions when antiviral therapy was discontinued or the dose was reduced.
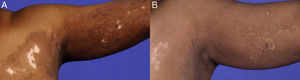
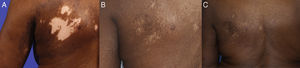
A 69-year-old African-American female presented in October 2002 with a one-month history of erythema, pruritus, and hypo- and hyperpigmentation that began on her left upper back and spread down her arm onto her left chest in a dermatomal distribution. She denied pain, anesthesia, or history of herpes zoster. Physical examination revealed hypo- and hyperpigmented patches on left upper back (9×8cm), left upper chest (8×7cm), and left arm (9×4cm) with 3% total body surface area (BSA) involvement (Fig. 1A; Fig. 2A). There were no bullae present. Wood's light examination showed depigmentation consistent with vitiligo.
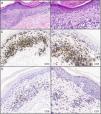
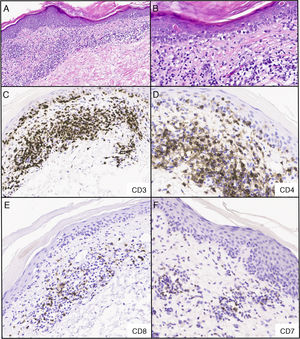
Biopsy of the left upper back showed a dermal CD4+and CD8+atypical lymphoid infiltrate with focal epidermotropism (Fig. 3A-B). Immunohistochemical studies (Fig. 3C-F) demonstrated CD3+T cells in the epidermis and dermis with predominance of CD4 over CD8 in the dermis, with a CD4:CD8 ratio of approximately 4:1. In the epidermis, there was a subset of atypical lymphocytes negative for CD4 and CD8. There is loss of CD7 expression. Rare cells were reactive for CD30. The majority of the lymphocytes were positive for TCR beta (BF1) and negative for TCR gamma. Monoclonal T-cell receptor gamma-chain gene rearrangements were detected by polymerase chain reaction, suggestive of MF. There were 42.2% of CD4+CD26- cells by flow cytometry, suspicious for CTCL involvement of the blood. The patient was given triamcinolone cream 0.1% and bexarotene gel after a diagnosis of stage IA MF.
Infiltrado linfoide dérmico atípico para las células CD4+y CD8+con epidermotropismo focal; los estudios inmunohistoquímicos realizados confirmaron presencia de células T CD3+en dermis y epidermis con predominancia de células CD4 sobre células CD8 en la dermis, en una proporción CD4:CD8 de, aproximadamente, 4:1. En la epidermis, se observó un subgrupo de linfocitos atípicos negativos para CD4 y CD8. Hay pérdida de expresión de CD7. Pocas células fueron reactivas al marcador CD30. La mayoría de los linfocitos dieron positivo para el receptor de la cadena beta de linfocitos T (BF1) y negativo para el receptor de la cadena gamma de linfocitos T. A) Tinción hematoxilina y eosina, 40x. B) Tinción hematoxilina y eosina, 200x C-F) inmunohistoquímica, 200x.
She demonstrated minimal improvement with topical agents. Because of the zosteriform pattern, the patient was given valacyclovir 1000mg daily. On extended antiviral therapy, her erythema and pruritus resolved within two months, leaving only pigmentary changes in a zosteriform distribution. Subsequently, she continued to receive valacyclovir, though the dose was halved to 500mg daily in January 2005.
By September 2006, 90% repigmentation was noted with maintenance therapy (Fig. 1B; Fig. 2B). However, the patient discontinued valacyclovir in May 2008 and one month later reportedly developed bullae with erythema, and so valacyclovir 500mg daily was resumed. She experienced additional flares in April 2009, May 2011, and May 2012, each about one month after an attempt to taper or discontinue the valacyclovir. On valacyclovir 500mg daily, she has maintained repigmentation with no additional flares in the last four years, with 0.75% BSA involvement at last follow-up in August 2016 (Fig. 2C).
A number of dermatoses may follow a dermatomal distribution. Although zoster and herpes simplex are the most common, MF should be considered in the differential diagnosis of zosteriform eruptions. Our patient represents the fourth case of zosteriform MF.1–3 Her dermatomal MF lesions might have occurred via lymphocytic invasion of perineural lymphatic vessels or the fenestrated vasculature of the dorsal root ganglion.4,5 Alternatively, she may have experienced recurrent zoster with histology mimicking MF, although the plausibility and frequency of recurrent zoster have been debated.6 She never developed vesiculobullous lesions that would suggest recurrent zoster. Furthermore, her lesions fulfilled the Pimpinelli algorithm for a diagnosis of early MF.7
In making a diagnosis of zosteriform MF, it is important to consider the histopathology given that it is a histopathological diagnosis. In the early lesions of vitiligo, there may lichenoid inflammation that can clinically and histopathologically mimic MF.8 There can be dense band-like lymphocytic infiltrates with exocytosis of cells into the lower layers of the epidermis.8 Since these lymphocytes are usually CD8+cytotoxic T cells, they can make a diagnosis of MF very difficult. Moreover, cutaneous herpes zoster and herpes simplex virus infection can present with atypical histologic features—dense lymphoid infiltrates, angiotropism, and atypical lymphocytes—that resemble MF.9 In these cases, the typical clinical and histopathological characteristics of viral infection may be absent, although histopathology may demonstrate the typical cytopathic changes of herpetic infection, which can be confirmed by PCR.9
Our case is extraordinary in that her MF and segmental vitiligo not only demonstrated a near-complete response to valacyclovir, but also, peculiarly, recurred after each of four attempts to taper or discontinue the medication. She represents the first case of a patient with zosteriform MF that responded to antiviral therapy. In addition, her condition improved over time on maintenance therapy since it was initiated in 2002. Her response to suppressive antiviral therapy, which poses minimal risks, spared her from the adverse effects of more advanced treatments.
Evidence of clinical activity of antiviral agents in MF remains very limited. Burg et al. and Resnick et al. found that, of 30 MF patients treated with acyclovir, one exhibited a complete response and six showed a partial response.10,11 Potential mechanisms for the effect of antiviral agents on MF are via direct cytopathic action or activation through thymidine kinase.10 Levin et al. noted that acyclovir has an inhibitory effect on the phytohemagglutinin blastogenic response of human blood mononuclear cells.12 In addition, antiviral agents act as a substrate for thymidine kinase, whose isoenzymes have high activity in peripheral mononuclear cells of some subtypes of lymphoma.10 Although their mechanism of action in MF remains unknown, antiviral agents may represent an appropriate treatment, particularly for its zosteriform variant.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Lewis DJ, Hinojosa T, Chan WH, Wu JH, Duvic M. Supresión de un cuadro de micosis fungoide recurrente con distribución zosteriforme mediante tratamiento de mantenimiento con valaciclovir. Actas Dermosifiliogr. 2018;109:757–760.