Voriconazole is a second-generation triazole antifungal agent that inhibits the enzyme cytochrome P450, which is required for the synthesis of ergosterol, a sterol that maintains the integrity of the fungal cell wall.1,2 In 2002 voriconazole was approved by the US Food and Drug Administration for the treatment of serious fungal infections including those caused by Fusarium and Aspergillus species.1 The main adverse effects of voriconazole are visual disturbances, elevated transaminase levels, gastrointestinal upset, and skin rashes, including photosensitivity, cheilitis, and xerosis.3,4 Prolonged use has been associated with the development of liver spots, actinic keratoses, squamous cell carcinoma (SCC), and even cutaneous melanoma.1 Most of the patients who develop SCC are immunocompromised (bone marrow, stem cell, or lung transplant recipients).5,6
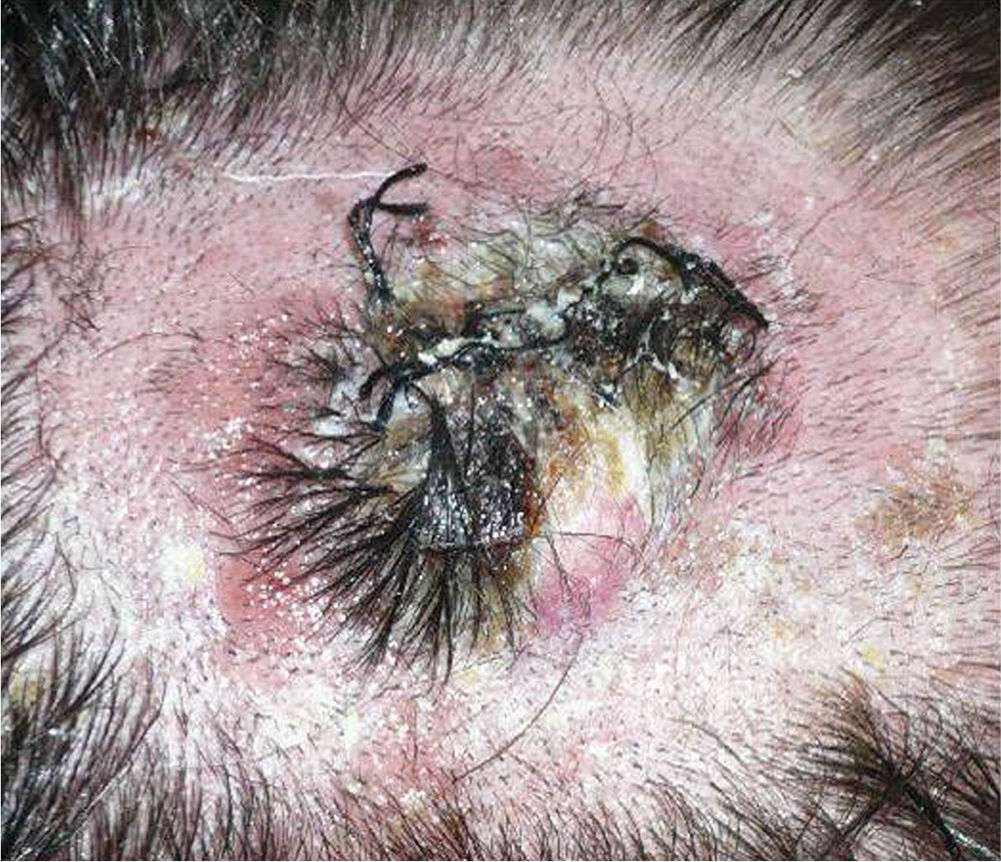
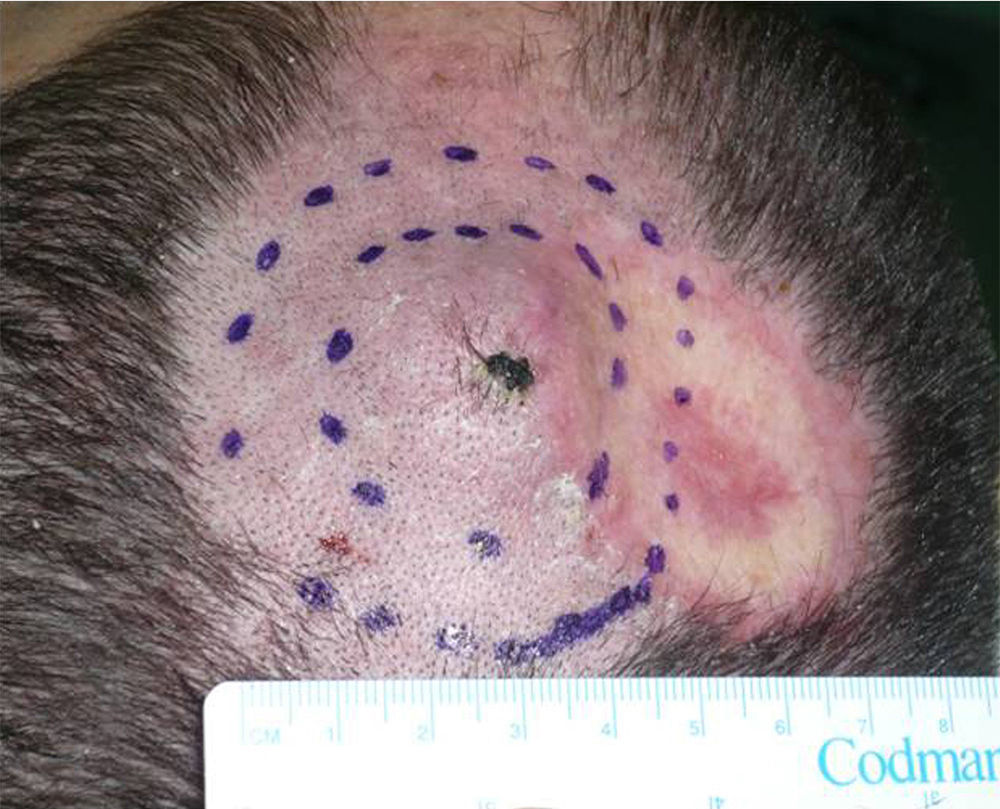
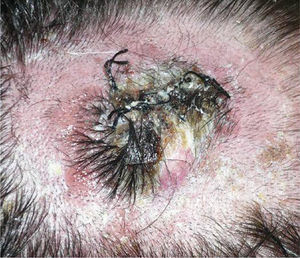
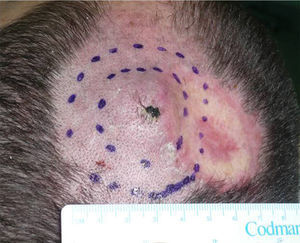
We describe the case of a 30-year-old man with Fitzpatrick skin phototype II and low sun exposure who had been diagnosed with cystic fibrosis. In 1998, at the age of 17, he underwent a double lung transplant. He was prescribed immunosuppressive therapy with tacrolimus, prednisone, and mycophenolate mofetil. In 2007, the patient underwent a right lower lobectomy for tuberculosis and pulmonary aspergillosis and began antifungal treatment with voriconazole. In 2011, after 3 years of treatment with voriconazole, he developed a fast-growing nodule in the left parietal region and multiple actinic keratoses on the rest of the scalp (Fig. 1). The tumor was excised by Mohs micrographic surgery and closed using a full-thickness skin graft. Histology revealed a moderately differentiated SCC (Fig. 2) with tumor-free margins. Voriconazole treatment was discontinued once the diagnosis was confirmed. Three months later, the patient developed a recurrence in the left parietal region, near the skin graft (Fig. 3). As tumor adhesion to the cortex was observed during resection of the lesion, the periosteum was excised and an osteotomy of the outer table performed. Weeks later, the patient developed left and right cervical lymph node metastases, for which he underwent bilateral lymph node dissection (4/29 positive nodes on the right side and 1/32 on the left) and subsequently received adjuvant radiotherapy at a dose of 50Gy (60Gy for level-II lymph node metastases). After 6 months free of disease, the patient developed a right cervical node recurrence with microscopic spread to surrounding soft tissues. The lesion was completely removed and the patient is currently awaiting adjuvant treatment.
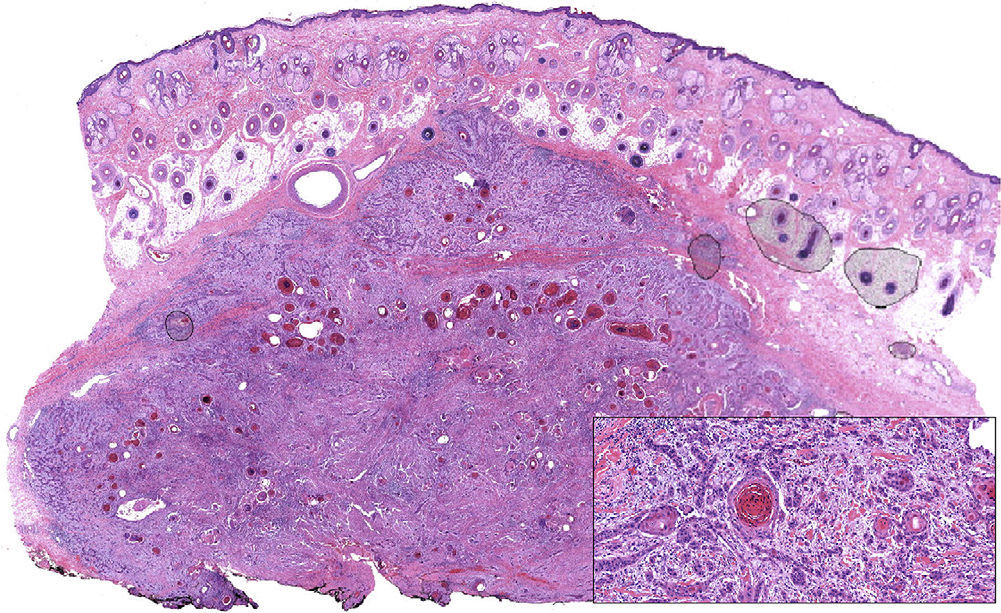
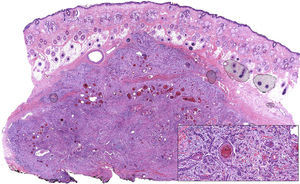
Moderately differentiated squamous cell carcinoma (hematoxylin eosin, original magnification ×10). The composition of the tumor, which consists of nests and strings of epithelial cells with extensive foci of keratotic differentiation, is shown in greater detail in the inset (hematoxylin eosin, original magnification ×400).
Skin tumors in patients with chronic immunosuppression are more common and usually more aggressive than in the general immunocompetent population. Furthermore, despite the small number of cases described in the literature, there is increasing evidence linking prolonged voriconazole treatment in immunocompromised patients with the development of multiple cutaneous SCCs that are more aggressive and more likely to recur locally than those observed in the general population.5–7 While the prolonged immunosuppression of the patient in the present case was likely an important factor influencing both the development and the aggressive course of the SCC, it is likely that prolonged treatment with voriconazole was the determining factor. To date, at least 18 cases of voriconazole-associated SCC have been described (2 children and 16 adults),8 all involving tumors in sun-exposed areas such as the face, neck, and dorsum of the hands. The scalp was affected in only in 6 these cases.
The mechanism that underlies the development of these tumors is unclear. A repeated, extensive, and intense phototoxic stimulus affecting sun-exposed areas may explain the appearance of preneoplastic lesions such as actinic keratoses, which subsequently progress to SCCs. Photocarcinogenesis is usually caused by cellular DNA damage, which in the present case was likely mediated by voriconazole and/or its metabolites, either directly or by oxidative stress.8
Particular care should be taken when prescribing voriconazole to immunosuppressed patients, especially if it is anticipated that the treatment will be long term or if the patient has a low phototype (I-II).2,8 It is recommended that these patents are closely monitored and take sun protection measures, which include avoiding sun exposure, wearing appropriate clothing, and using suncream.2,4 In some cases it may be necessary to discontinue voriconazole treatment. The possibility of substituting voriconazole with another antifungal agent with a suitable spectrum (especially posaconazole or itraconazole) should also be considered.2
Please cite this article as: Carrascosa R, Solano-López GE, Vargas E, Fraga J. Carcinoma espinocelular en un paciente inmunosuprimido en tratamiento con voriconazol. Actas Dermosifiliogr. 2014;105:424–426.