Solar urticaria is a chronic inducible urticaria also classified as an idiopathic dermatosis. The objective of this paper is to define the phenotypic characteristics of solar urticaria and to evaluate its incidence.
Material and methodThis was a retrospective multicenter study in which data were gathered on the epidemiology and clinical, photobiologic, laboratory, and therapeutic characteristics of solar urticaria.
ResultsA total of 224 patients (141 women and 83 men) were included from 9 photobiology units. The mean age of the patients was 37.9 years (range, 3-73 years). A history of atopy was detected in 26.7%, and the most common presentation was allergic rhinitis (16.5%). Clinical signs were limited to sun-exposed areas in 75.9% of patients. The light spectrum most commonly implicated was visible light only (31.7%), and in 21% of cases it was only possible to trigger solar urticaria with natural light. The treatments most widely used by photobiology experts were oral antihistamines (65.46%), followed by different forms of phototherapy (34%). Complete resolution was observed most often in patients with solar urticaria triggered exclusively by visible or natural light, with statistically significant differences with respect to other wavelengths (P<.05). No increase in the annual incidence of solar urticaria was observed.
ConclusionsWe have presented the largest series of solar urticaria published to date. The epidemiological, clinical, and photobiologic findings confirm previously reported data, although there was a particularly high rate of negative phototests in our series. Reactivity exclusively to visible or natural light was associated with a higher probability of resolution. No increasing trend was observed in the annual incidence.
La urticaria solar es una urticaria crónica inducible física clasificada también como fotodermatosis idiopática. El objetivo de este trabajo es definir las características fenotípicas y valorar su incidencia.
Material y métodoEstudio multicéntrico retrospectivo en el que recogen datos epidemiológicos, características clínicas, fotobiológicas, analíticas y terapéuticas.
ResultadosSe ha incluido a 224 pacientes procedentes de 9 Unidades de Fotobiología. La distribución por sexos correspondió a 141 mujeres y 83 varones con una edad media al diagnóstico de 37,9 años (rango 3-73). El 26,7% presentaba antecedentes de atopia, con la rinitis alérgica como la manifestación más frecuente (16,5%). Un 75,9% de los pacientes refería clínica solo en zonas fotoexpuestas. El espectro implicado con más frecuencia fue la luz visible aisladamente (31,7%). En el 21% la urticaria solar solo fue posible desencadenarla con luz natural. El tratamiento más empleado por los expertos fueron los antihistamínicos por vía oral (65,46%) seguido por diferentes modalidades de fototerapia (34%). La resolución completa se observó con mayor frecuencia en urticaria solar desencadenada exclusivamente por luz visible o luz natural, con diferencias estadísticamente significativas (p<0,05) con respecto a otras longitudes de onda. No se observa un incremento de la incidencia anual.
ConclusionesPresentamos la serie de urticaria solar más larga hasta ahora publicada. Las características epidemiológicas, clínicas y fotobiológicas confirman los datos ya conocidos, aunque en nuestra serie destaca un alto índice de fototest negativos. La reactividad exclusiva a luz visible o luz natural se asocia a mayores probabilidades de resolución. No se observa una tendencia al aumento en la incidencia anual.
Solar urticaria (SU) is an uncommon idiopathic photodermatosis that accounts for 7% of all photodermatoses.1 It is also classified as a chronic inducible (physical) urticaria,2 and in this context, it accounts for between 0.08%1 and 0.4%3 of all urticarias. It primarily affects women in the third decade of life and is characterized by the appearance of pruritus, erythema, or wheals in sun-exposed areas generally within an hour of exposure to sun or artificial light. Manifestations disappear within a maximum of 24hours of cessation of exposure.4
Most cases of SU follow a benign course, but some patients develop systemic symptoms, such as headache, nausea, mucosal involvement, and even anaphylaxis. Whether these symptoms are present or not, SU can negatively impact the personal and even professional life of those affected.5
A patient's clinical history is usually sufficient to reach a diagnosis of SU. Recent guidelines, however, recommend additional evaluation by a photobiology unit,2 as a photobiological study can confirm diagnosis, identify the wavelength or wavelengths responsible for inducing SU, and help to select the most appropriate treatment depending on the severity of the condition.
Different treatment modalities have been used depending on the intensity of the accompanying symptoms, and include sunscreens, oral antihistamines,6 ciclosporin,7 desensitization with various forms of phototherapy,8,9 omalizumab,10 plasmapheresis,11 intravenous immunoglobulin therapy,12 and afamelanotide.13 Although treatment recommendations have been made for chronic urticaria, and more recently for chronic inducible urticaria, consensus- and evidence-based treatment guidelines are lacking for SU.2
Numerous case reports and series published over the years1,14–22 and recently summarized in a review article22 have helped to improve our understanding of the characteristics of SU.
In this study we present the largest series of SU published to date. The series includes 20 cases already reported on in a preliminary study.20 We describe the epidemiological, clinical, laboratory, and photobiological characteristics of SU with the aim of better defining the phenotypic characteristics of this photodermatosis.
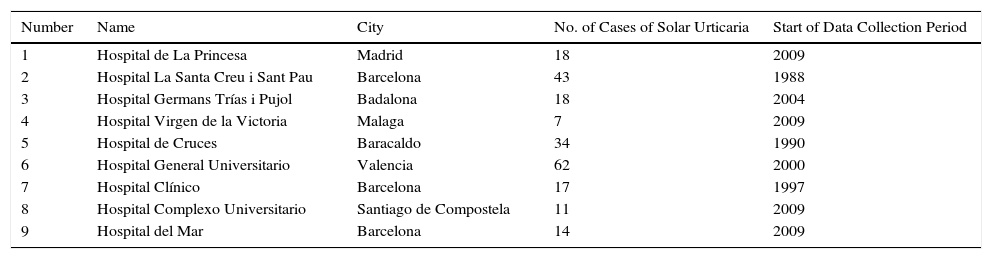
Material and MethodsStudy DesignThis was a multicenter retrospective study undertaken by the Spanish Photobiology Group (known by its Spanish acronym GEF). The authors are all heads of photobiology units in tertiary hospitals located in different parts of Spain (Table 1).
Participating Hospitals.
| Number | Name | City | No. of Cases of Solar Urticaria | Start of Data Collection Period |
|---|---|---|---|---|
| 1 | Hospital de La Princesa | Madrid | 18 | 2009 |
| 2 | Hospital La Santa Creu i Sant Pau | Barcelona | 43 | 1988 |
| 3 | Hospital Germans Trías i Pujol | Badalona | 18 | 2004 |
| 4 | Hospital Virgen de la Victoria | Malaga | 7 | 2009 |
| 5 | Hospital de Cruces | Baracaldo | 34 | 1990 |
| 6 | Hospital General Universitario | Valencia | 62 | 2000 |
| 7 | Hospital Clínico | Barcelona | 17 | 1997 |
| 8 | Hospital Complexo Universitario | Santiago de Compostela | 11 | 2009 |
| 9 | Hospital del Mar | Barcelona | 14 | 2009 |
Patients were assessed according to the routine practices of each unit and informed consent for diagnostic procedures and interventions was obtained according to the policies at each hospital.
The data collection period varied from one center to the next, but in all cases, data were collected up to June 2014.
Study PopulationInclusion criteria: patients of either sex and any age who at the time of evaluation had signs or symptoms of SU or natural or artificial light provocation test results consistent with a diagnosis of SU.
The following data were collected for each patient: sex, age at the time of diagnosis, personal history, skin phototype, time since onset of SU, clinical and photobiological characteristics of SU, treatments used, follow-up time, and clinical course.
Photobiological StudyPhototests were applied to the lumbar region or the anterior aspect of the forearm of all patients and results were read immediately after the tests and at 30 and 60minutes. When a positive response was observed for any of the variables tested, the first dose that induced the erythema was considered positive. The doses used for the photoprovocation tests varied, as most of the patients had been evaluated before publication of GEF's phototesting consensus document23 and before calibration of the equipment used across the photobiology units. For this reason, information on minimal urticarial doses is not provided in this article.
The following sources of light were used: 1) solar simulators equipped with a xenon arc lamp (Solar Light 12S and 16S, Solar Light and Oriel Solar Simulator 300W, Newport) and 2) broadband UV-B fluorescent lamps. The most widely used models were Philips TL-12 (Medisun Gigatest, Schulze & Böhm GmbH) and Waldmann UV21 (Waldmann UV 180 and 181, Waldmann).
Three types of lamps were used for exposure to UV-A radiation: 1) a solar simulator with a cut-off filter that eliminates wavelengths below 320nm, 2) UV-A fluorescent lamps (Waldmann UV181 devices with Waldmann PUVA lamps), and 3) high-pressure UV-A lamps (Waldmann UVA700 and Philips HB400).
A Kodak slide carousel with a 150-W halogen bulb placed at 10cm from the skin with a maximum illumination time of 10minutes was used to test visible light.
Patients with a negative phototest result were asked to expose a small area of skin to natural light for the time it typically took for symptoms to appear and to return to the unit to evaluate the response.
Laboratory TestsThe following laboratory tests were performed in all patients: complete blood count, differential white blood cell count, blood biochemistry, and determination of antinuclear antibodies (ANAs) and plasma protoporphyrins. Total immunoglobulin (Ig) E levels were also measured in a subset of patients.
Statistical AnalysisThe characteristics of the patients and study variables are presented as frequencies. The χ2 test was used to compare proportions to estimate degrees of disproportion. Qualitative variables were compared using parametric tests (analysis of variance, Snedecor's F test, and the t test). Finally a Cox regression model was used to estimate the probability of clinical resolution and present the results as Kaplan-Meier curves.
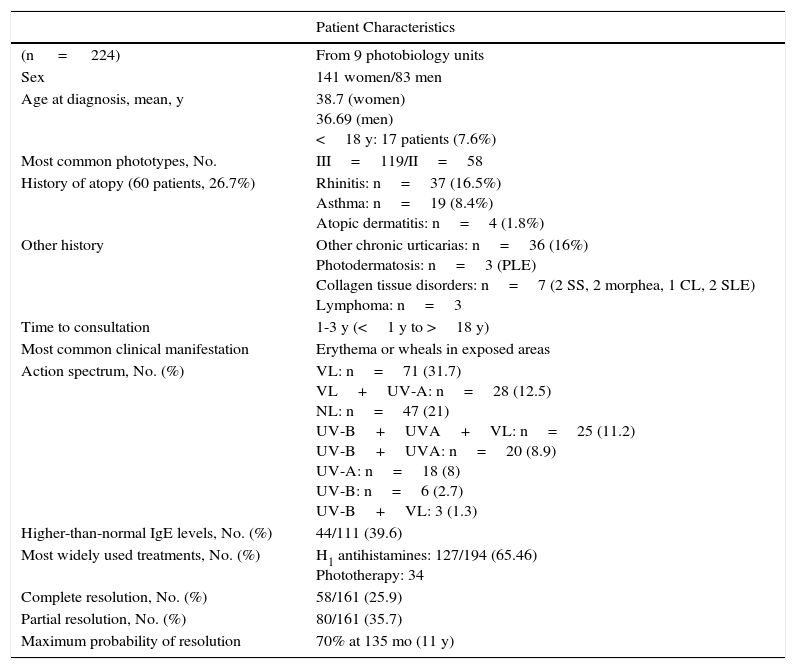
ResultsIn total, 224 patients from 9 photobiology units were included in the study. The main results are summarized in Table 2. The number of cases pertaining to each center varied considerably depending on the activity of the unit and the length of the data collection period (Table 1).
Main Study Findings.
| Patient Characteristics | |
|---|---|
| (n = 224) | From 9 photobiology units |
| Sex | 141 women/83 men |
| Age at diagnosis, mean, y | 38.7 (women) 36.69 (men) <18 y: 17 patients (7.6%) |
| Most common phototypes, No. | III=119/II=58 |
| History of atopy (60 patients, 26.7%) | Rhinitis: n=37 (16.5%) Asthma: n=19 (8.4%) Atopic dermatitis: n=4 (1.8%) |
| Other history | Other chronic urticarias: n=36 (16%) Photodermatosis: n=3 (PLE) Collagen tissue disorders: n=7 (2 SS, 2 morphea, 1 CL, 2 SLE) Lymphoma: n=3 |
| Time to consultation | 1-3 y (<1 y to >18 y) |
| Most common clinical manifestation | Erythema or wheals in exposed areas |
| Action spectrum, No. (%) | VL: n=71 (31.7) VL+UV-A: n=28 (12.5) NL: n=47 (21) UV-B+UVA+VL: n=25 (11.2) UV-B+UVA: n=20 (8.9) UV-A: n=18 (8) UV-B: n=6 (2.7) UV-B+VL: 3 (1.3) |
| Higher-than-normal IgE levels, No. (%) | 44/111 (39.6) |
| Most widely used treatments, No. (%) | H1 antihistamines: 127/194 (65.46) Phototherapy: 34 |
| Complete resolution, No. (%) | 58/161 (25.9) |
| Partial resolution, No. (%) | 80/161 (35.7) |
| Maximum probability of resolution | 70% at 135 mo (11 y) |
Abbreviations: CL, cutaneous lupus; NL, natural light; PLE, polymorphic light eruption; SLE, systemic lupus erythematosus; SS, Sjörgen syndrome; VL, visible light.
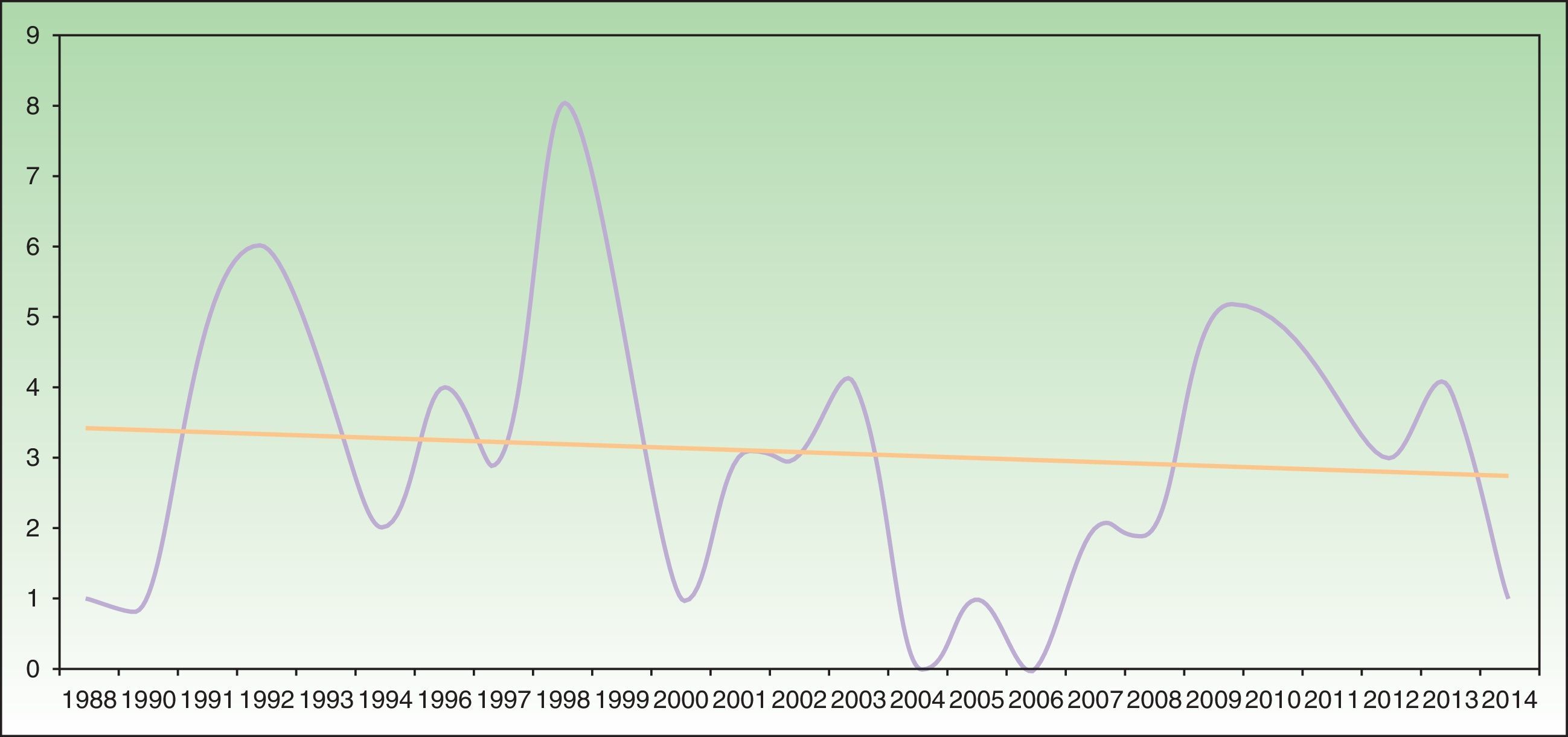
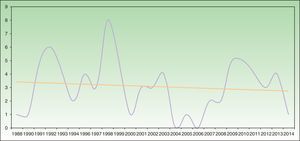
Analysis of SU incidence (number of new cases diagnosed yearly) in the 2 centers with the longest follow-up times did not reveal a tendency towards an increase in annual incidence over time (Figure 1).
Annual incidence of solar urticaria between 1998 and 2014 at Hospital La Santa Creu i Sant Pau in Barcelona (43 cases) and Hospital de Cruces en Baracaldo (34 cases). The mean number of cases was approximately 3 cases per year with a slight trend towards a reduction in incidence, as shown by the trend curve.
There were 141 women and 83 men in the study group. Mean age at diagnosis was 37.9 years (range, 3-73 years) for the overall group, 38.7 years (range, 3-73 years) for women, and 36.69 years (range, 5-73 years) for men. Seventeen patients (7.6%) were younger than 18 years at the time of diagnosis. The most common skin phototypes were type II (n=58) and type III (n=119).
Personal HistorySixty patients (26.7%) had a past history of atopy; the most common manifestation was allergic rhinitis (n=37, 16.5%), followed by allergic asthma (n=19, 8.4%). Only 4 patients had a history of atopic dermatitis. Other forms of chronic urticaria, including dermographism (factitial urticaria), were detected in association with SU in 36 patients (16%). Seven patients had collagen disorders (2 cases each of Sjögren syndrome, morphea, and systemic lupus erythematosus and 1 case of cutaneous lupus). Three patients had polymorphous light eruption and another 3 were diagnosed with lymphoma (diffuse large B-cell lymphoma, follicle center lymphoma, and primary cutaneous marginal zone B-cell lymphoma) during follow-up of SU.
Clinical FormsTime from the onset of symptoms to consideration for evaluation at the photobiology unit was 1 to 3 years (range, 1 to >18 years).
In total, 75.9% of patients developed erythema or wheals accompanied by pruritus in sun-exposed areas within minutes of sun exposure. Urticaria affecting skin covered by thin clothing during sun exposure was reported by 15.6% of patients. Face and hand involvement was additionally reported by 6.3%. One patient reported SU manifestations affecting areas of bruising or trauma only. Delayed SU was observed in 1 patient, who developed lesions in exposed and non exposed areas several hours after sun exposure. In another patient, the SU resolved on interruption of triflusal. No other cases linked to concomitant medication were reported. Finally, 1 patient experienced anaphylactic symptoms and syncope while exposed to sun at the beach.
Photobiological TestingPhotobiology test results were available for 218 of the 224 patients. The most frequently involved action spectrum was visible light, which alone was responsible for manifestations in 71 patients (31.7%). UV-A and visible light were implicated in 28 patients (12.5%). UV-B, UV-A, and visible light were identified as causes of SU in 25 patients (11.2%), and UV-B and UV-A were identified in 20 patients (8.9%). Eighteen patients (8%) reacted to UV-A only, and the corresponding rates for UV-B and for UV-B and visible light were 2.7% (6 patients) and 1.3% (3 patients). Finally, 47 patients (21%) reacted only to natural light.
Laboratory FindingsIgE levels were measured in 111 of the 224 patients, and 44 (39.6%) had values above normal (100U/mL). Mean IgE was 234U/mL (range, 2.7-2.874U/mL). Men with higher-than-normal IgE levels were on average younger than women (31.14 vs 40.9 years) and than men with normal IgE levels and men without an IgE result. The difference was statistically significant (P<.05).
Five patients had significant ANA titers (≥1/320). One had cutaneous lupus, another had systemic lupus erythematosus, and 3 did not have an autoimmune disease.
TreatmentData on treatments used were available for 194 of the 224 patients. Treatments varied from one hospital to the next according to clinician experience and prescribing practices. The most common treatments were oral H1 antihistamines, which were used either alone or in combination with other treatments in 65.46% of patients (127/194). Phototherapy was employed in 34% of cases. The different forms of phototherapy were heliotherapy (17.77%), UV-A (8.12%), psoralen plus UV-A (4.06%), and narrowband UV-B (4.06%).
Other treatments mentioned were sunscreens, beta-carotene, antimalarials, and immunosuppressants. Omalizumab was used in 4 patients, 3 of whom responded favorably.
Follow-upInformation on clinical outcomes was available for 161 of the 224 patients. Complete resolution of lesions, whether spontaneous or induced by treatment, was reported for 58 patients (25.9%), while partial resolution was reported for 80 (35.7%). Twenty-three patients (10.3%) had experienced no improvement by the end of the data collection period. Follow-up duration ranged from less than 1 year to over 20 years, but the most common period was 1 to 3 years (n=109, 48.7%).
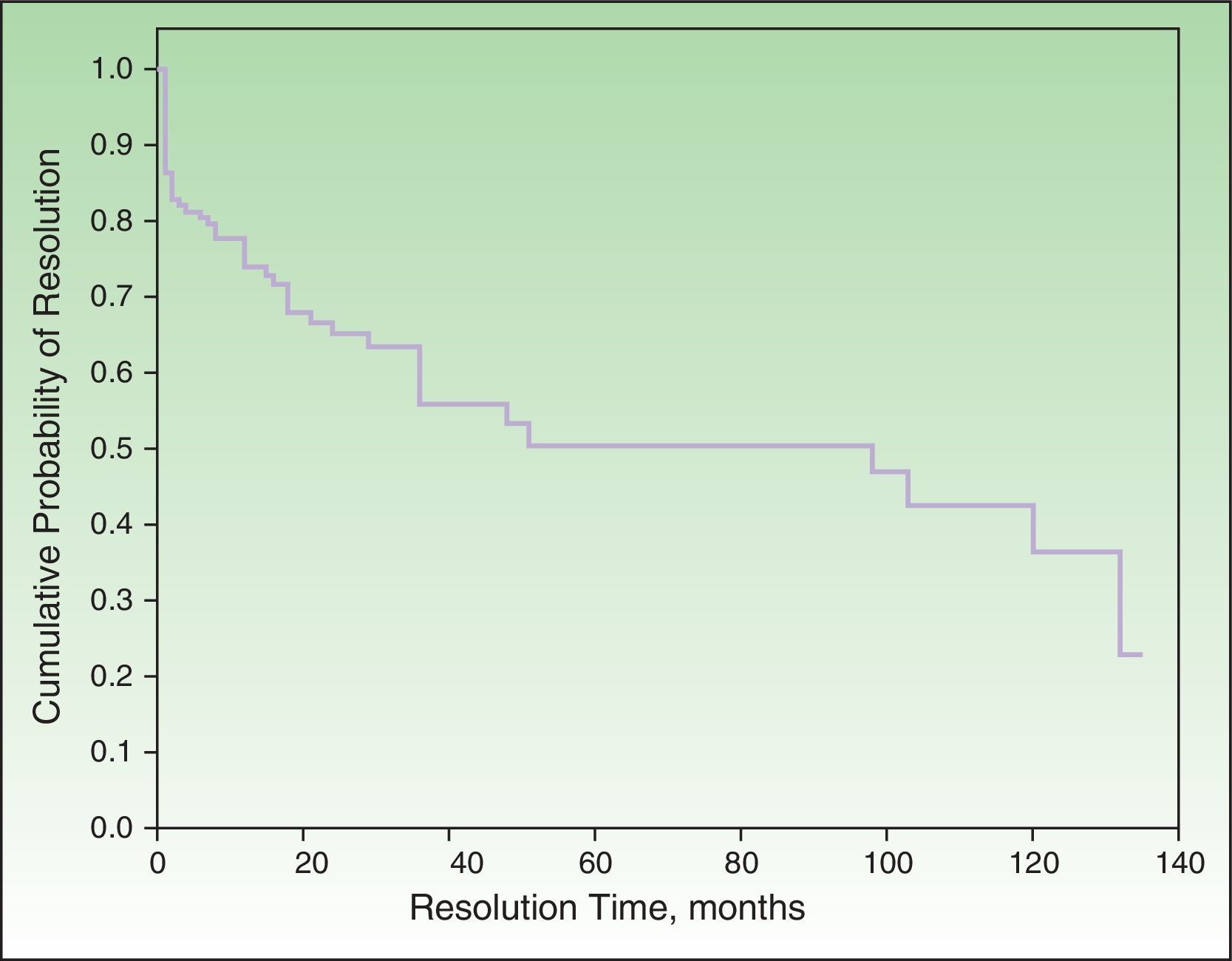
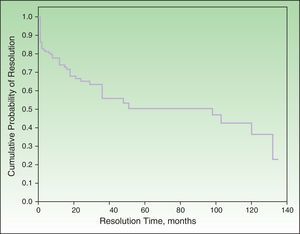
Probability of ResolutionThe maximum probability of resolution after 135 months (11.5 years) of follow-up was 70%. Half of the cases had resolved after 4 to 5 years of follow-up (Figure 2).
Multivariate AnalysisComplete resolution was more common in patients in whom SU was induced exclusively by visible or natural light. The differences were statistically significant (P<.05) with respect to the other wavelengths. Patients who experienced partial resolution or no improvement at all were more likely to react to both visible light and UV-A.
Half of the patients who achieved complete response did so in the first year of follow-up. After 3 years, this percentage had increased to 83%. Complete resolution was more common in men without a history of atopy, although the difference was not statistically significant.
DiscussionWe have presented the epidemiological, clinical, laboratory, and photobiological characteristics of SU based on a chart review of 224 patients evaluated by 9 photobiology units at 9 hospitals in different parts of Spain. The heads of these units are all members of the GEF.
Ten series describing 370 patients with SU from different countries have been published to date,1,14–22 and 1 of these reported on 20 patients included in the current series.20
SU is a rare photodermatitis with an unknown etiology and pathogenesis. The most recent classification system proposed by Leenutaphong et al.24 is based on in vitro activation studies and divides SU into 2 types: type I, caused by an abnormal chromophore present only in patients with SU, and type II, caused by abnormal circulating IgE antibodies directed against a normal chromophore.
Some recent publications have reported a trend that suggests an increase in the incidence of SU (although the possible reasons remain unidentified),22,25 together with a reduction in the incidence of actinic reticuloid.25 We cannot confirm this trend based on our data, as the temporal distribution of SU incidence in the 2 hospitals with the longest follow-up periods showed a slight trend towards a reduction in incidence (Figure 1).
The sex and age distribution of the patients in the current series is similar to that already described in the literature. The only series to report a predominance of male sex is a series from Singapore.1 In all the studies published to date, the third and fourth decades of life were the most common periods for onset of SU. The incidence of SU in patients younger than 18 years was 7.8% in our series, contrasting with reports of 5% to 15% for patients under 15 years14,16 and 21% to 27% for those under 20 years.14,16,17 We agree that the main phototypes involved in SU will vary according to geographic area.1,22 In our series, the most common type was phototype III, followed by phototype II; this is consistent with skin types described for the healthy population in Spain.23
The incidence of atopy in patients with SU varies from one series to the next, with rates ranging from 0% to 48%.14,15 However, none of the series to date have specified the types of atopy involved. We observed a history of atopy in 26.7% of the patients in our series, and this is consistent with reports from other Mediterranean countries.18 Rhinitis and allergic asthma were the most common manifestations in our series, and there were very few cases of atopic dermatitis. The incidence of concomitant chronic urticarias (16% in our series) has only been reported in 3 series to date, with rates ranging between 5% and 34%.1,17,22
The low incidence of concomitant photodermatosis in our series coincides with most reports to date.14,21,22 Just 3 patients in our series had polymorphous light eruption in association with SU. The series described by Beattie et al.16 is the only one to date to report a considerable incidence of both conditions (23% of patients with SU had polymorphous light eruption). The association between SU and connective tissue disorders and lymphoma detected in our series appears to be incidental and highlights the importance of thorough history taking and follow-up in patients with SU.
The clinical manifestations of SU are easy to recognize. In all the series described to date, most patients reported the development of erythema or wheals with pruritus in sun-exposed areas within minutes of exposure. The mean time from onset of symptoms to evaluation at a photobiology unit was 1 to 3 years in our series. This implies that there was an equivalent delay in determining the characteristics of the patients’ SU and consequently prescribing adequate treatment. Some patients took over 18 years to consult about their condition, and similar diagnostic delays have been reported.14,15,18,20,22
The most common wavelength implicated in SU in our series was visible light, either alone or associated with UV-A. Again this coincides with the majority of previous reports.14,16–18,20,21 The high proportion of negative phototests in our series (21%) is noteworthy. Although negative results have been reported in the literature, the rates have been lower (0%-3.5%).1,14–17,20 There are several possible explanations for the high rates of negative phototests observed in our series. First, not all data were available for all patients due to the retrospective nature of the study. Second, a substantial proportion of patients came for evaluation at a time of the year when they were free of symptoms (which occur only in certain seasons). Third, the doses required to achieve a positive result may have been higher than those used in the photobiology units. It is also possible that certain patients may have had SU induced by the simultaneous action of several spectral bands and would therefore only have reacted to exposure to sunlight and not to individual bands emitted by the test devices.
Our findings also show altered plasma IgE levels. Although IgE was measured in just 50% of patients, we believe that our findings are relevant as approximately 40% of the patients tested had above-normal levels; this rate is similar to that reported for other types of chronic urticaria (36%).26 We do not know whether the younger mean age of the subset of male patients with high IgE levels is relevant or not. However, we do believe that regular IgE measurements are important as SU is an IgE-mediated disease.
The enormous variability in treatment options identified in our series can be explained by the absence of standardized treatment guidelines and the fact that choices were determined by the experience of the different clinicians. Our results suggest that oral H1 antihistamines are the treatment of choice in most hospitals. The patients’ records did not specify whether or not the doses had been escalated up to 4 times the recommended dose as H1 antihistamine dose escalation in such settings is a very recent recommendation.27,28 Perhaps though, the time has come to consider treating SU as if it were another form of chronic urticaria. The latest recommendations for the management of chronic inducible urticaria support this idea,2 and based on a systematic review of the evidence, show that the use of nonsedating H1 antihistamines is supported by level A evidence. The use of desensitization phototherapy or afamelanotide is supported by level B evidence, while that of omalizumab, ciclosporin, or intravenous immunoglobulin therapy is supported by level C evidence.
Omalizumab was used to treat 4 patients in our series, and 3 of these responded favorably with the doses currently recommended for chronic urticaria. The literature shows varying results for the effectiveness of omalizumab in the treatment of SU.10,29 In our opinion, controlled multicenter studies are necessary to draw more definitive conclusions regarding the use of this drug in SU.
None of the factors studied in the multivariate analysis had a significant influence on the resolution or nonresolution of SU. We only saw that patients who reacted exclusively to visible light or natural light were significantly more likely to achieve complete resolution compared with those who reacted to other light spectrum regions. Complete resolution was achieved by 25.9% of patients in our series. The equivalent rates reported by Beattie et al.16 and Monfreccola et al.17 were 25% and 74%, respectively. The maximum probability of resolution in our series was 70% after 135 months (11 years). Beattie et al. reported resolution rates of 12%, 26%, and 36% for 5, 10, and 15 years, respectively.
In conclusion, the epidemiological, clinical, and photobiological characteristics of SU in our series of 224 patients, the largest published to date, are similar to most of the findings reported in the literature. One particularly noteworthy finding is that we did not detect a trend towards an increase in annual incidence. Rhinitis and asthma were the most common atopic manifestations and chronic urticaria in association with SU was not uncommon. The high rate of negative phototests compared with other series is interesting. Patients who reacted exclusively to visible light or natural light were more likely to achieve resolution of SU than those who reacted to other portions of the spectrum. H1 antihistamines were the most widely used treatment in our series, followed by phototherapy. Nevertheless, due to a lack of clear evidence-based recommendations for the treatment of SU, it would be interesting to conduct multicenter studies to help identify an adequate treatment protocol for this photodermatosis.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors thank all those who directly or indirectly contributed to the production of this manuscript, particularly Dr Antonio Salazar Cifre, epidemiologist at the Department of Epidemiology at the Centro de Salud Pública in Valencia, and Dr Noelia Rivera and Dr José Ramón Estela Cubells, residents at Hospital Germans Trías i Pujol in Badalona and Hospital General de Valencia, respectively, for their help during the data collection phase of this study.
Please cite this article as: Pérez-Ferriols A, Barnadas M, Gardeazábal J, de Argila D, Carrascosa JM, Aguilera P, et al. Urticaria solar. Epidemiología y fenotipos clínicos en una serie española de 224 pacientes. Actas Dermosifiliogr. 2017;108:132–139.