Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) is an emerging pathogen that causes skin and soft-tissue infections.
ObjectiveTo describe the clinical characteristics of skin infections caused by CA-MRSA and correlations with the available demographic and microbiological data.
Material and methodsThis was a descriptive study of patients with a microbiologically confirmed diagnosis of CA-MRSA infection treated in a dermatology department between June 2009 and December 2011. We recorded demographic details, the clinical characteristics of lesions, and the treatments used.
ResultsWe studied 11 patients (5 men and 6 women); 91% were under 40 years of age and had no relevant past medical history. The most common presentation was a skin abscess (with or without cellulitis). In all such cases, marked tissue necrosis and little or no purulent exudate was observed when the abscess was drained. Fifty percent of these abscesses had been treated previously with β-lactam antibiotics, and in all cases the lesions resolved after surgical drainage, which was combined in 63% of cases with quinolones or cotrimoxazole.
ConclusionsToday, skin infections due to CA-MRSA affect healthy young athletes who have no contact with healthcare settings. The most common presentation is a skin abscess characterized by marked tissue necrosis and little or no purulent exudate. In cases with these characteristics in susceptible patients, the involvement of CA-MRSA as the causative agent should be suspected. The abscesses should be drained whenever possible and, if necessary, antibiotic treatment should be prescribed; empirical use of β-lactam antibiotics should be avoided.
Staphylococcus aureus resistente a meticilina de adquisición comunitaria (SARM-CO) constituye un patógeno emergente como agente productor de infecciones de piel y partes blandas.
ObjetivoDescribir las características clínicas de las infecciones cutáneas causadas por SARM-CO, así como su correlación con los datos demográficos y microbiológicos disponibles.
Material y métodosSe realizó un estudio descriptivo de los pacientes con diagnóstico de infección microbiológicamente confirmada por SARM-CO en el Servicio de Dermatología desde junio de 2009 a diciembre de 2011. Se recogieron los datos demográficos de los pacientes, las características clínicas de las lesiones y los tratamientos realizados.
ResultadosSe reclutaron 11 pacientes, 5 hombres y 6 mujeres. El 91% tenía menos de 40 años y no presentaba antecedentes médicos de interés. El absceso cutáneo (asociado o no a celulitis) fue la forma más frecuente de presentación. Tras su drenaje se observó una cantidad escasa o nula de exudado purulento junto a un componente de necrosis tisular marcado en todas estas lesiones. El 50% de los abscesos había recibido tratamiento previo con antibióticos betalactámicos y el drenaje quirúrgico, asociado en el 63% a quinolonas o cotrimoxazol, aseguró la resolución de los mismos.
ConclusionesActualmente las infecciones cutáneas causadas por SARM-CO se presentan en individuos jóvenes, sanos, deportistas, sin contacto con el ámbito sanitario. Los abscesos cutáneos, su forma más frecuente de presentación, se caracterizan por una marcada necrosis tisular, con escaso o nulo exudado purulento. Ante estos hallazgos en pacientes susceptibles debe sospecharse la implicación de SARM-CO como agente causal. El drenaje de los abscesos se realizará siempre que sea posible, y cuando resulte necesario añadir tratamiento antibiótico deberá evitarse el uso de betalactámicos de forma empírica.
The increase in infections caused by microorganisms resistant to conventional antimicrobials represents a major therapeutic limitation in the hospital setting.
Soon after the introduction of penicillin in 1941 the first strains of penicillin-resistant Staphylococcus aureus were isolated. Hospital strains of methicillin (oxacillin)-resistant S aureus appeared 2 decades later, and subsequently became one of the main bacterial causes of nosocomial infections. However, since the 1980s S aureus has also been detected in non-hospital-acquired infections,1 and its incidence has grown exponentially in many countries, including the United States, where it is the leading cause of community-acquired skin and soft tissue infections in several states.2
Community-acquired methicillin-resistant S aureus (CA-MRSA) differs from its nosocomial counterpart in its clinical, epidemiological, and microbiological characteristics (Table 1),3–7 and thus requires radically different therapeutic management in most cases.
Differential Characteristics of Nosocomial and Community-Acquired MRSA Strains.
| Community-Acquired MRSA | Nosocomial MRSA | |
| Risk groups/factors | Children, athletes, membership of recruitment institutions, sexual promiscuity, IVDAs, HIV,4 tattoos,5,6 origin in endemic area (Pacific Islanders, Central/South Americans, indigenous Australians) | Prolonged hospitalization, intravascular catheters, diabetes mellitus, immunosuppression, admission to intensive care unit, hemodialysis, peritoneal dialysis |
| Mechanisms of resistance (SCCmec genes) | IV | I, II, III |
| Production of Panton-Valentine leukocidin | Frequent | Infrequent |
| Antimicrobial resistance pattern | Resistance to β-lactam antibiotics | Multidrug resistance |
| Clinical symptoms | Infections of skin and soft tissue, necrotizing pneumonia | Bacteremia associated with endovascular devices, urinary tract infection, nosocomial pneumonia, endocarditis, surgical wound infection |
| Colonization | Genital, perineal (recurrent infections) | Nasal |
Abbreviations: HIV, human immunodeficiency virus; IVDAs, intravenous drug addicts; MRSA, methicillin-resistant Staphylococcus aureus; SCCmec, staphylococcal cassette chromosome.
Source: Kowalski et al.3
aIn Spain CA-MRSA isolates frequently exhibit resistance to tetracycline and doxycycline mediated by the tet(M) gene.7
Given that CA-MRSA predominantly affects skin and soft tissue, we reviewed 11 cases diagnosed in our hospital in order to describe the main clinical features of the disease and the correlation with available demographic and microbiological data. Based on our findings, we propose several specific clinical criteria to improve the initial empirical treatment of skin and soft tissue infections in outpatients.
Materials and MethodsThis is a descriptive, observational, retrospective study of adult patients with a microbiological diagnosis of skin and soft tissue infection caused by CA-MRSA. The data were collected between June 2009 and December 2011 from the skin infection database of the dermatology department at the Hospital General Universitario Gregorio Marañón in Madrid, Spain.
All cases had been microbiologically confirmed by culture followed by antibiogram and molecular analysis.
The clinical variables studied were age, sex, clinical presentation (lesion type, presence of pus, necrosis, and location), the course of the lesions, treatments received, and the response to treatment. We analyzed the origin of the patients, the presence of recurrent skin infections in the patient's family, risk practices (contact sports or activities, shared use of personal hygiene items, membership of recruiting groups or institutions), contact with the health sector (employment, previous hospital admissions), and contact with domestic or farm animals.
ResultsData for the patients studied are summarized in Table 2.
Demographic, Clinical, and Microbiological Data for Patients Included in the Study.
| Patient/Age/Sex | Origin | Lesion Type | Lesion Location | Purulent Exudate | Tissue Necrosis | Risk Factors | Prior Treatments | NBA Resistance | PVL Production | Treatment |
| 1/38/M | Spain | Abscess + cellulitis | Leg | No | Yes | Sport (futsal) | Cloxacillin, amox-clav | Erythromycin | Yes | SD + oral ciprofloxacin |
| 2/40/M | Ecuador | Abscess | Arm | No | Yes | Erythromycin | Yes | SD | ||
| 3/27/M | Spain | Abscess | Leg | No | Yes | Camping in rural Thailand, | No | Yes | SD + oral ciprofloxacin | |
| 4/26/F | Cuba | Abscess | Iliac fossa | Scarce | Yes | dance, tattoo | Cloxacillin, amox-clav, cefuroxime | Erythromycin, levofloxacin | Yes | SD + oral clindamycin (administered in suspension due to digestive intolerance)/cotrimoxazole |
| 5/43/M | Colombia | Abscess | Abdomen | Scarce | Yes | Amox-clav | Erythromycin | Yes | SD + IV vancomycin | |
| 6/37/F | Spain | Anthrax | Upper lip | Scarce | No | Amox-clav | No | Yes | SD + oral ciprofloxacin | |
| 7/35/M | Poland | Paronychia | Big toe | Scarce | No | Ingrown toenail | Amox-clav, cefuroxime | No | Yes | Oral cotrimoxazole + deferred SD of ingrown toenail |
| 8/37/F | Poland | Abscess + cellulitis | Thigh | No | Yes | Sports (swimming, diving) | No | Yes | SD + oral cotrimoxazole | |
| 9/18/F | Ecuador | Abscess + cellulitis | Abdomen | No | Yes | Noninvasive cosmetic procedures | Erythromycin | Yes | SD + oral ciprofloxacin | |
| 10/18/F | Colombia | Folliculitis | Back | Yes | No | Recurrent skin infections | Erythromycin | Yes | Cotrimoxazole | |
| 11/24/F | Spain | Abscess + cellulitis | Leg | Scarce | Yes | Dance | Amox-clav, erythromycin | Erythromycin, clindamycin | SD + vancomycin |
Abbreviations: Amox-clav, amoxicillin-clavulanate; F, female; IV, intravenous; M, male; NBA, non-β-lactam antibiotics; PVL, Panton-Valentine leukocidin; SD, surgical drainage.
Of the patients studied, 91% (10/11) were younger than 40 years, and the male to female ratio was 1:1.2. The individuals were healthy, immunocompetent patients with no prior hospital admissions and no contact with the healthcare sector through either employment or family.
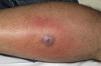
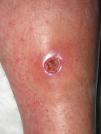
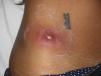
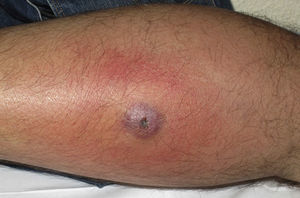
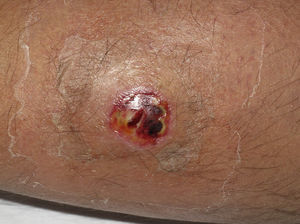
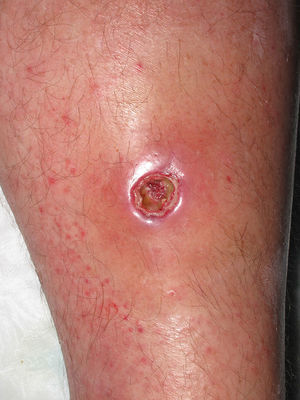
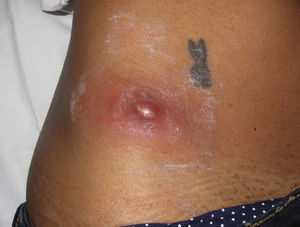
Eight (73%) of the 11 patients presented with abscesses (Figs. 1-4); half of these cases (4/8) involved perilesional cellulitis, and 63% (5/8) presented abscesses on exposed areas of the limbs. Surgical drainage removed small amounts of purulent material in 37% (3/8) of cases, and none in the remainder (63%; 5/8). Tissue necrosis, forming a cavity within the subcutaneous tissue, was the main feature of all lesions.
Primary follicular involvement (staphylococcal folliculitis and anthrax) was observed in 2 patients, and recurrent paronychia in another. Due to the nature of the lesions, it was not possible to clearly establish the amount of purulent exudate in these 3 patients.
Thirty-six percent (4/11) of the patients were Spanish, 45% (5/11) were Latin American, and 18% (2/11) were Polish. Possible risk factors associated with the development of infection included sports (2 patients, 18%), dance (2 patients, 18%), and noninvasive cosmetic procedures (1 patient, 9%).
Two patients had a history of recurrent, uncomplicated skin infections.
All cases had been confirmed by culture followed by an antibiogram and molecular analysis. The bacterial strains isolated in 64% (7/11) of the cases studied were resistant to erythromycin. Levofloxacin resistance was also detected in 1 case (1/7) and inducible clindamycin resistance in another (1/7), both of which were confirmed by D-test. Genes encoding Panton-Valentine leukocidin (PVL) were expressed in all staphylococcal isolates analyzed (except 1 case in which no analysis was performed as the patient came from another health care center).
Of the patients analyzed, 55% (6/11) had been previously treated with amoxicillin-clavulanate with no clinical response. Of these, 67% (4/6) experienced treatment failure after a second round of antibiotic therapy with β-lactam (cefuroxime/cloxacillin) or erythromycin.
All abscesses (8/8) were surgically drained and antibiotics administered in 88% of cases. Ciprofloxacin and cotrimoxazole were the drugs of choice in 73% of patients (8/11). In 1 case treatment with oral clindamycin was discontinued due to gastrointestinal intolerance (diarrhea). Two patients with uncomplicated abscesses were treated with intravenous vancomycin as an alternative to β-lactam antibiotics. None of the patients presented systemic complications or myofascial involvement.
DiscussionCA-MRSA is emerging as a causative agent of skin and soft tissue infections. Its incidence remains low in Spain, in contrast to other industrialized countries, where it constitutes one of the main community-acquired bacterial pathogens.8,9
The epidemiological data presented here are similar to those reported in the scientific literature: those affected are young, healthy patients who engage in contact sports/activities or come from endemic areas such as Central or South America.9
Often, patients present single abscesses, located in areas of skin that are exposed or subject to contact during sports/activity.10 Tissue necrosis is the main characteristic of these abscesses and very little pus is produced. A study evaluating the presence of eschar and perilesional cellulitis as clinical predictors in abscesses caused by CA-MRSA reported a sensitivity of 92% and a positive predictive value of 94%.11 These findings may be explained by the cytotoxic effects that PVL is thought to exert on neutrophils and epidermal cells, and by the release of proinflammatory factors secondary to granulocyte lysis.12 However, further studies are needed to confirm the role of this toxin in the pathogenesis of infections caused by CA-MRSA.
The differential diagnosis of these lesions should include pyoderma gangrenosum, spider bites,13 loxoscelism,14 and cutaneous leishmaniasis.
Other common cutaneous manifestations have been described, including folliculitis, carbuncles (recurrent infections), and impetigo (especially in children).15
CA-MRSA strains have microbiological characteristics that differentiate them from nosocomial strains; they are usually resistant only to β-lactam antibiotics and carry the genes that encode the PVL subunits LukF-PV and LukS-PV. However, depending on the source of the strain CA-MRSA may be also resistant to other antibiotics, as is the case with the predominant clone in the USA, USA300 (ST8-MRSA-IV), which is highly resistant to macrolides.7 Therefore, where possible, samples should be taken in all cases for microbiological analysis of the lesions.
In this series 91% of patients received antimicrobial therapy, combined with surgical abscess drainage. However, some authors claim that lesion drainage and daily local treatment is sufficient in cases of extrafacial abscesses of less than 5cm in diameter in nondiabetic, immunocompetent patients without myofascial involvement.16–18Table 3 summarizes the various relevant therapeutic modalities for the treatment of infections caused by CA-MRSA.
Therapeutic Modalities in Infections Caused by CA-MRSA.
| Treatment | Indications | Dosea/Route of Administration | Adverse Effects and Precautions | Comments |
| Surgical drainage | Should be performed for all abscesses | |||
| Cotrimoxazole | 1. Abscesses> 1 cmor < 1cm in case of infants, patients over 65 y, facial location, diabetics, immunosuppression, perilesional cellulite2. Impetigo, ecthyma, cellulitis, folliculitis, paronychia | OralAdults and patients ≥ 12 y: 800/160 mg/12 hChildren:6-12 y, 400/80 mg/12 h6 mo-5 y, 200/40 mg/12 h6 wk-5 mo, 100/20 mg/12 hIV /IMAdults and patients > 12 y: 800/160 mg/12-24 hChildren 6-12 y: 400/80 mg/12-24 hMean dose: 80/16mg /5 kg/12 hChildren > 2 mo: 6-12 mg/kg/d trimethoprim | Toxicodermia, leukopenia, thrombocytopenia, hemolytic anemia, megaloblastic anemia, photosensitivityNot for use in case of pregnancy, lactation, infants < 2 mo, glucose-6-phosphate dehydrogenase deficiency, porphyrias, treatment with methotrexate, combination with azathioprine or metformin | High rate of resistance in infections caused by Streptococcus pyogenes: caution advised in the empirical treatment of abscesses with perilesional cellulitis |
| Clindamycin | 1. Abscesses> 5 cm or< 5cm in case of infants, patients over 65 y, facial location, diabetics, immunosuppression, perilesional cellulite2. Impetigo, ecthyma, cellulitis, folliculitis, paronychia | OralAdults: 150-450 mg/6 hChildren > 1 mo: 8-25 mg/kg/d in 3-4 dosesIV /IMAdults: 1.2-1.8 g/d in 3-4 dosesChildren > 1 mo: 20-40 mg/kg/d in 3-4 doses | Diarrhea, risk of pseudomembranous colitis, unpleasant metallic tasteShould not be used in cases of pregnancy, lactation, or in combination with erythromycin or aminoglycosides | Can presentresistance that is indetectable with standard antibiograms (confirm that D-test has been performed if conventional antibiogram indicates erythromycin resistance) |
| Quinolones (ciprofloxacin, levofloxacin) | 1. Abscesses> 5 cmor < 5cm in case of infants, patients over 65 y, facial location, diabetics, immunosuppression, perilesional cellulite2. Impetigo, ecthyma, cellulitis, folliculitis, paronychia | CiprofloxacinaOral: Adults, 500 mg/12 hIV: Adults, 200-400 mg/12 hLevofloxacinaAdults: Oral, 250-500 mg/dIV, 500 mg/d | Risk of tendinopathy, arthropathy (greater in children, the elderly, and in patients receiving long-term corticosteroid therapy)Adjust dose in cases of renal impairmentAvoid in cases of pregnancy, lactation. Should not be ingested with dairy products or calcium | Rates of resistance to these drugs are currently low19 |
| Tetracyclines(Doxycycline, minocycline) | 1. Abscesses> 5 cmor < 5cm in case of infants, patients over 65 y, facial location, diabetics, immunosuppression, perilesional cellulite2. Impetigo, ecthyma, cellulitis, folliculitis, paronychia | DoxycyclineaOral: Adults and children > 8 y (weight ≥ 45 kg), 100-200 mg/24 h in 1 or 2 dosesMinocyclineaOral: Adults and children > 8 y (weight ≥ 45 kg), 100 mg/12 h | Photosensitivity, hepatotoxicity, benign intracranial hypertension (increased risk when administered in children or with retinoids), vestibular toxicity (minocycline), esophagitisAvoid in cases of pregnancy, lactation, children < 8 y (risk of tooth and bone metabolism disorders) | Not effective in monotherapyThe ST80-MRSA-IV strains (present in Northern and Eastern Europe and in some countries of the Mediterranean basin) are resistant to tetracycline |
| Rifampicin | 1. Abscesses> 5 cmor < 5cm in case of infants, patients over 65 y, facial location, diabetics, immunosuppression, perilesional cellulite2. Impetigo, ecthyma, cellulitis, folliculitis, paronychia | OralAdults: 450-600 mg/dChildren > 5 y: 10-20 mg/kg/d in 1-2 doses (maximum 600 mg/d) | Hepatotoxicity, changes in color of urine/bodily secretions, multiple drug interactions (cytochrome p450 inducer) | Synergistic effect with other antimicrobials. Do not use in monotherapy (frequent emergence of resistant strains) |
| Daptomycinb | Complicated skin and soft tissue infections | IVAdults: 4-6mg /kg/dSafety and efficacy not established in patients < 18 y | MyotoxicityDosage adjustment in case of renal diseaseAlterations in hemostatic parameters (INR/prothrombin time) | Do not use if there is a risk of necrotizing lung infection (action inhibited by pulmonary surfactant) |
| Vancomycinb | Complicated skin and soft tissue infections | IVAdults: initial dose, 30-50mg /kg/d; typical dose, 1 g/12 h or 500 mg/6 hChildren > 1 mo: 10 mg/kg/6 h | Nephrotoxicity. Red man syndrome: avoid rapid intravenous administration (minimum 1 h) | Monitor serum levels in patients with renal failure or in cases of coadministration with nephrotoxic drugs |
| Linezolidb | Complicated skin and soft tissue infections | Oral and IVAdults: 600 mg/12 hNot recommended in patients < 18 y | Myelosuppression, tyramine reaction, optic nerve neuropathy, hypertension, elevated transaminase levels | Given its high cost and adverse effects, its use must be strictly warranted. |
| Quinupristin-dalfopristinb | Complicated skin and soft tissue infections | Administered through central venous catheter in 5% glucose for 60 min: 7.5 mg/kg/8 h (not diluted in saline) | Increased QT interval, hepatotoxicity, increased plasma levels of drugs metabolized by CYP3A4 | Should be used only in case of treatment failure with other available alternatives |
Abbreviations: AHT, arterial hypertension; INR, international normalized ratio; IM, intramuscular; IV, intravenous.
There is no consensus on the duration of administration regimens for the majority of these drugs. In general, it is recommended to re-evaluate after 7 days of treatment and extend treatment to 14 days if necessary (treatment with quinupristin-dalfopristin should not be extended for more than 7 days).
Since this bacterium mainly causes uncomplicated skin infections, treatment does not usually require hospitalization. However, cases of septic arthritis, pyomyositis, fascitis,20 and necrotizing pneumonia21 have been described. Necrotizing pneumonia21 should be considered in patients with uncomplicated skin infections and past or simultaneous symptoms of respiratory infection.22 These cases always require antimicrobial treatment and close clinical observation.
The determination of carrier status by nasal swab is not recommended as a regular procedure.16 Nasal colonization is often low in patients with lesions that are not associated with community-acquired MRSA outbreaks (the majority of cases in Spain), and the bacteria may be detected in other locations such as the inguinal/perineal area, the axillary folds, the rectum, or the pharynx.23 There is no consensus on the measures necessary to achieve decolonization of this bacteria,24 although some authors have proposed showering with antiseptic soap solutions (chlorhexidine or povidone iodine, although use of the latter is limited by its staining of the skin and the risk of local sensitization or irritation25), or using topical intranasal (mupirocin) and systemic (cotrimoxazole) antibiotics,26,27 with varying results.
ConclusionsUncomplicated infections of the skin and soft tissue are one of the main reasons for visits to dermatological practices and emergency health services. The epidemiology of these infections has changed in recent years, with the appearance of pathogens that were hitherto considered exclusively nosocomial. CA-MRSA represents a paradigm in dermatological practice, as its main pathogenic targets are the skin and skin adnexa. The collection of samples for culture and subsequent antibiogram remains the gold standard in diagnosis. However, while awaiting the results of microbiological tests, we believe it is essential to individualize the initial empirical antibiotic treatment and to include this pathogen in the bacterial spectrum covered.
Although the small number of cases analyzed and the retrospective nature of our study do not allow us to generalize based on the results obtained, we propose that the use of β-lactam antibiotics as an initial therapeutic measure should be avoided in young, healthy patients with cutaneous abscesses with a significant necrotic component and scant purulent exudate, with or without cellulitis. Similarly, in cases of recurrent skin infections, family outbreaks of abscesses, folliculitis, and carbuncles, or treatment failure after 1 or more cycles of β-lactam antibiotics, CA-MRSA should be considered as a potential causative pathogen.
Ethical DisclosuresProtection of Human and Animal SubjectsThe authors declare that no experiments were performed on humans or animals during the course of this study.
Confidentiality of DataThe authors declare that they have followed the protocols of their place of work concerning the publication of patient data and that all patients included in the study were appropriately informed and gave their written informed consent.
Right to Privacy and Informed ConsentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pulido Pérez A, Baniandrés Rodríguez O, Ceballos Rodríguez MC, Mendoza Cembranos MD, Campos Domínguez M, Suárez Fernández R. Infecciones cutáneas causadas por Staphylococcus aureus resistente a meticilina de adquisición comunitaria: características clínico-microbiológicas en 11 pacientes. Actas Dermosifiliogr. 2014;105:150–158.