Defining quality indicators is a key strategy for ensuring the quality and standardization of health care. The CUDERMA project, an initiative of the Spanish Academy of Dermatology and Venerology (AEDV), was undertaken to define quality indicators for the certification of specialized units in dermatology; the first 2 areas selected were psoriasis and dermato-oncology. The aim of this study was to reach a consensus on what should be assessed by the indicators used to certify psoriasis units. The structured process used to do this comprised a literature review to identify potential indicators, the selection of an initial set of indicators to be evaluated by a multidisciplinary group of experts and, finally, a Delphi consensus study. A panel of 39 dermatologists evaluated the selected indicators and classified them as either “essential” or “of excellence”. Consensus was finally reached on 67 indicators, which will be standardized and used to develop the certification standard for psoriasis units.
La definición de indicadores de calidad es una estrategia clave para garantizar la calidad de la asistencia sanitaria y su homogenización. Así, el proyecto CUDERMA surge como una iniciativa de la AEDV para definir indicadores de calidad con los que certificar unidades de distintos campos de interés en la dermatología, de los que se seleccionaron psoriasis y dermatooncología de forma inicial. El objetivo de este trabajo fue consensuar los aspectos a evaluar por los indicadores en la certificación de las unidades de psoriasis. Para ello se siguió un proceso estructurado que contempló la revisión bibliográfica de indicadores, la elaboración de un set preliminar revisado por un grupo de expertos multidisciplinar y el consenso Delphi. Un panel de 39 dermatólogos evaluó los indicadores, y los clasificó como «básicos» o «de excelencia». Finalmente se consensuaron 67 indicadores que serán estandarizados para diseñar la norma con la que certificar las unidades de psoriasis.
Psoriasis is a common, chronic, recurrent, immune-mediated disease1 that can result in significantly impaired quality of life2 as it interferes with daily activities3 and is associated with psychologic4,5 and cardiovascular3,6 comorbidities that increase mortality risk.
Dermatology units specialized in the management of psoriasis have a crucial role in providing care to patients.7 Although these units are characterized by high-quality services, standardization of processes and practices would be desirable to reduce the variations identified across the different regions of Spain8 and guide further pursuit of excellence in care.
Certification of specialized units is an increasingly popular strategy for standardizing and improving the quality of health care.9–11 Apart from reducing variations in care provision and ensuring minimum quality levels, certification also promotes the transfer of professional knowledge into practical, relevant, and up-to-date care based on the latest trends in clinical practice.9,10 Certification uses objective criteria that establish the basic tenets of patient care. These criteria are evaluated by certification bodies during official audits consisting of inspections (e.g., of facilities, protocols, and results) and interviews with unit staff. If these predefined criteria are met, the unit receives official certification.9
A range of approaches exist for defining clinical audit criteria, or standards. Quality indicators are a well-established tool for this purpose, as they show the extent to which a unit's facilities, resources, and performance guarantee a minimum standard of care. Quality indicators can also be used to assess a unit's activity and identify areas with room for improvement.12,13
Indicators used to date to evaluate quality of care in psoriasis have been based on level of compliance with clinical practice guidelines.14 In Spain, several studies have developed quality indicators for the certification of atopic dermatitis,15 psoriatic arthritis,11,16–18 and dermato-oncology19,20 units. Quality indicators for use in dermatology have also been developed in other countries.21,22
The Certification of Dermatology Care Units (CUDERMA) project was launched by the Spanish Academy of Dermatology and Venereology (AEDV) in collaboration with the academy's Psoriasis Group to develop quality indicators for the certification of specialized dermatology units in Spain. The project has 3 phases. The goal of the first phase is to identify and reach consensus on which aspects need to be evaluated by the quality indicators. In the second phase, these aspects are unified and standardized, with establishment of names, definitions, standards, objective criteria for compliance, and evidence of compliance. In the third and final phase, the newly defined standard will be used to certify units.
The aim of this study was to achieve consensus on which aspects should be measured by quality indicators for the certification of psoriasis units.
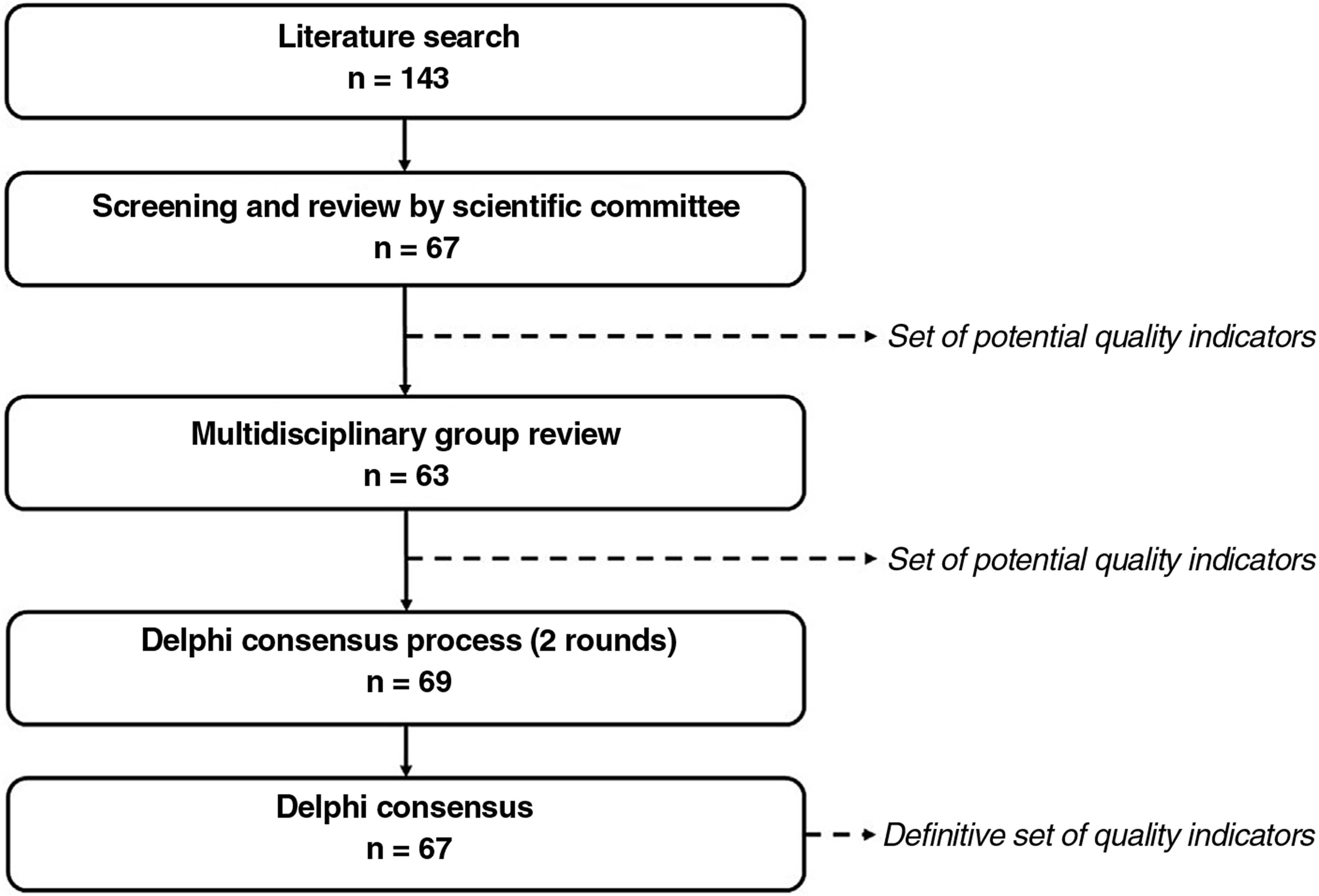
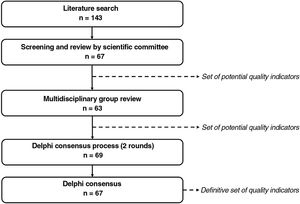
Material and MethodsSelection of the aspects to be measured by the quality indicators took place in 3 phases: 1) identification of potential indicators; 2) review by a multidisciplinary group of experts and generation of a preliminary list of indicators; and 3) a Delphi consensus process to agree on relevant aspects to evaluate for the certification of psoriasis units (Fig. 1).
The study was led by a working group formed by the 4 members of the CUDERMA coordinating group and a scientific committee of 6 dermatologists with experience in the management of patients with psoriasis (Table A.1), all members of the AEDV. The group received support from 3 methodology experts.
Phase 1: Identification of Potential Quality IndicatorsPotential indicators were identified via a structured literature search based on the Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) framework (Table A.2).
The search yielded publications containing information on relevant aspects to evaluate during the certification of psoriasis units. The texts were reviewed to identify and extract potential indicators.
The working group reviewed these indicators based on clinical criteria specific to the management of psoriasis and proposed other possibilities. The resulting list was divided into 3 groups: structural indicators (to assess essential characteristics of a psoriasis unit), process indicators (to assess the unit's activity), and outcome indicators (to assess the results of this activity).12 Each group was further divided into thematic subgroups. This list constituted the preliminary set of potential quality indicators.
Phase 2: Multidisciplinary Group ReviewIn phase 2, the set of potential indicators was analyzed by a multidisciplinary group of 10 experts (Table A.1): 1 cardiometabolic specialist, 2 nurses, 1 hospital pharmacist, 1 family doctor, 1 preventive medicine specialist, 2 patients (1 from a patient's association), and 2 rheumatologists.
The multidisciplinary group rated the relevance of each indicator using a purpose-designed questionnaire containing sections for adding comments, suggesting modifications, and proposing new indicators.
The answers were evaluated by the working group, which then drew up a preliminary set of indicators to present to the Delphi panel.
Phase 3: Delphi Consensus ProcessThe Delphi technique is a methodology designed to achieve consensus among a group of participants who complete a series of individual questionnaires. These participants form what is known as a Delphi panel. The Delphi technique is an iterative procedure that takes place over several rounds in which the panelists rate a series of items on a Likert scale and add any comments they see opportune. Each person's answers are then processed to generate personalized questionnaires for the second round; the answers from this round are used to determine the final level of consensus for each item.23–25
Using the above framework, the preliminary set of indicators was presented to a Delphi panel formed by dermatologists from the AEDV's Psoriasis Group (Table A.1). The task of the panel was to achieve consensus over 2 rounds on which aspects should be evaluated when certifying a psoriasis unit.
In the first round, the panelists completed a questionnaire containing the name and definition of each indicator. Working individually, they rated each indicator on a scale of 1–9 (where 1 indicated not relevant and 9 very relevant). They were also asked to classify each indicator as “essential” (aspects that are essential to the functioning of a psoriasis unit) or “of excellence” (aspects that add value but if absent do not interfere with the unit's activity or performance). Panelists were also able to add comments, suggest changes, and propose new indicators.
The answers from the first round were processed to check that the indicators had been correctly understood. The working group also reviewed suggestions for new indicators for inclusion in the second round.
The group then used the answers from the first round to design individualized questionnaires for the second round. These included the scores and classifications given to each indicator in the first round, the mean scores for the group as a whole, and the percentage of panelists who classified a given indicator as “essential”. No new proposals for quality indicators were accepted in this round.
The answers from the second round of the Delphi consensus process were analyzed using the RAND/UCLA Delphi panel method, which uses statistical methods to provide a summary of opinions and calculate the level of consensus for each item.26 The median score for each indicator was placed in 1 of 3 regions: 1–3, 4–6, or 7–9. The first step was to determine the level of agreement for each indicator and determine whether the panelists agreed on its inclusion or exclusion.
Panelists were considered to agree on items scored within the median region by at least two-thirds of the panelists. When at least one-third scored the item within the 1–3 region and another third or more scored it within the 7–9 region, they were considered to disagree. All other indications were rated as “indeterminate” (neither agreement nor disagreement).
Indicators rated as “agreed on” or “indeterminate” were then assessed to determine whether the panelists were in favor or against their inclusion. Inclusion was considered “appropriate” for indicators with a median score in the region of 7–9 and “inappropriate” for those with a median score in the region of 1–3. Indications for indicators with a score in the range of 4–6 were considered “uncertain”.
Mean (SD) scores were also calculated to characterize the panelists’ responses.
The RAND/UCLA Delphi panel method was also used to determine whether a given indicator should be classified as “essential” or “of excellence”.26 Consensus was considered to have been achieved when more than two-thirds of the panelists assigned the same classification to a given indicator. In all other cases, the indications were classified as indeterminate.
Finally, the working group reviewed all indicators classified as uncertain or indeterminate or that the panelists had disagreed on and decided whether to include or exclude them and whether they were “essential” or “of excellence”.
ResultsPhase 1: Identification of Potential Quality IndicatorsThe literature search yielded 185 publications (Fig. A.1). Of these, 128 were eliminated after initial screening. The remaining 57 articles were analyzed in a full-text review, which produced a preliminary list of 143 potential indicators.27–84
The working group reviewed these indicators and narrowed the list down to 67: 21 structural indicators, 42 process indicators, and 4 outcome indicators.
Phase 2: Multidisciplinary Group ReviewThe review by the multidisciplinary group helped classify the indicators and in some cases improve their definition.
The relevance scores led to the elimination of 4 indicators, leaving a total of 63: 7 structural indicators, 52 process indicators, and 4 outcome indicators.
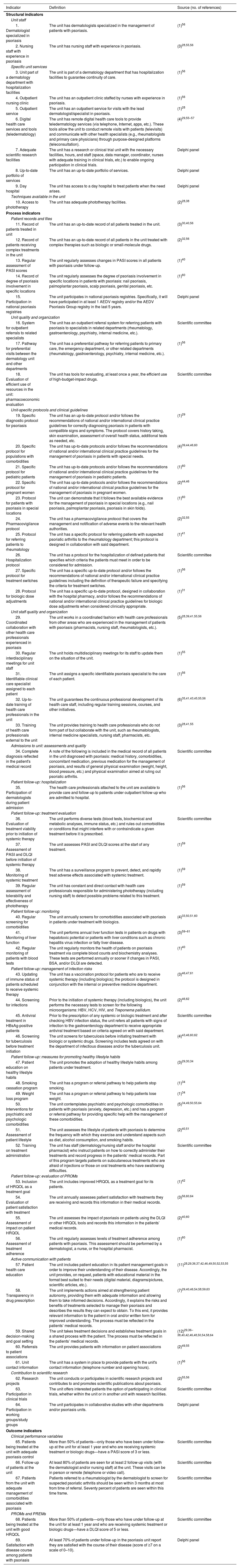
Phase 3: Delphi Consensus ProcessForty-two dermatologists participated in the first round of the Delphi consensus process to analyze the preliminary set of 63 indicators (Table 1) approved by the multidisciplinary group.
Quality Indicators Presented to the Delphi Panel.
| Indicator | Definition | Source (no. of references) |
|---|---|---|
| Structural indicators | ||
| Unit staff | ||
| 1. Dermatologist specialized in psoriasis | The unit has dermatologists specialized in the management of patients with psoriasis. | (1)56 |
| 2. Nursing staff with experience in psoriasis | The unit has nursing staff with experience in psoriasis. | (3)28,55,56 |
| Specific unit services | ||
| 3. Unit part of a dermatology department with hospitalization facilities | The unit is part of a dermatology department that has hospitalization facilities to guarantee continuity of care. | (1)56 |
| 4. Outpatient nursing clinic | The unit has an outpatient clinic staffed by nurses with experience in psoriasis. | (1)56 |
| 5. Outpatient service | The unit has an outpatient service for visits with the lead dermatologist/specialist in psoriasis. | (1)28 |
| 6. Digital health care services and tools (teledermatology) | The unit has remote digital health care tools to provide teledermatology services (via telephone, Internet, apps, etc.). These tools allow the unit to conduct remote visits with patients (televisits) and communicate with other health specialists (e.g., rheumatologists and primary care physicians) through purpose-designed platforms (teleconsultation). | (4)29,55–57 |
| 7. Adequate scientific research facilities | The unit has a research or clinical trial unit with the necessary facilities, hours, and staff (space, data manager, coordinator, nurses with adequate training in clinical trials, etc.) to enable ongoing participation in clinical trials. | Delphi panel |
| 8. Up-to-date portfolio of services | The unit has an up-to-date portfolio of services. | Delphi panel |
| 9. Day hospital | The unit has access to a day hospital to treat patients when the need arises. | Delphi panel |
| Techniques available in the unit | ||
| 10. Access to phototherapy | The unit has adequate phototherapy facilities. | (2)28,38 |
| Process indicators | ||
| Patient records and files | ||
| 11. Record of patients treated in unit | The unit has an up-to-date record of all patients treated in the unit. | (3)30,40,56 |
| 12. Record of patients receiving complex treatments in the unit | The unit has an up-to-date record of all patients in the unit treated with complex therapies such as biologic or small-molecule drugs. | (2)32,56 |
| 13. Regular assessment of PASI scores | The unit regularly assesses changes in PASI scores in all patients with psoriasis under follow-up. | (1)40 |
| 14. Record of degree of psoriasis involvement in specific locations | The unit regularly assesses the degree of psoriasis involvement in specific locations in patients with psoriasis: nail psoriasis, palmoplantar psoriasis, scalp psoriasis, genital psoriasis, etc. | (1)60 |
| 15. Participation in national psoriasis registries | The unit participates in national psoriasis registries. Specifically, it will have participated in at least 1 AEDV registry and/or the AEDV Psoriasis Group registry in the last 5 years. | Delphi panel |
| Unit quality and organization | ||
| 16. System for outpatient referrals to related specialists | The unit has an outpatient referral system for referring patients with psoriasis to specialists in related departments (rheumatology, gastroenterology, psychiatry, internal medicine, etc.). | Scientific committee |
| 17. Pathway for preferential visits between the dermatology unit and other departments | The unit has a preferential pathway for referring patients to primary care, the emergency department, or other related departments (rheumatology, gastroenterology, psychiatry, internal medicine, etc.). | (1)56 |
| 18. Evaluation of efficient use of resources in the unit: pharmacoeconomic evaluation | The unit has tools for evaluating, at least once a year, the efficient use of high-budget-impact drugs. | Scientific committee |
| Unit-specific protocols and clinical guidelines | ||
| 19. Specific diagnostic protocol for psoriasis | The unit has an up-to-date protocol and/or follows the recommendations of national and/or international clinical practice guidelines for correctly diagnosing psoriasis in patients with compatible signs and symptoms. The protocol covers history taking, skin examination, assessment of overall health status, additional tests as needed, etc. | (1)29 |
| 20. Specific protocol for populations with comorbidities | The unit has up-to-date protocols and/or follows the recommendations of national and/or international clinical practice guidelines for the management of psoriasis in patients with special needs. | (4)39,44,46,60 |
| 21. Specific protocol for pediatric patients | The unit has up-to-date protocols and/or follows the recommendations of national and/or international clinical practice guidelines for the management of psoriasis in pediatric patients. | (1)60 |
| 22. Specific protocol for pregnant women | The unit has up-to-date protocols and/or follows the recommendations of national and/or international clinical practice guidelines for the management of psoriasis in pregnant women. | (2)44,46 |
| 23. Protocol for patients with psoriasis in special locations | The unit can demonstrate that it follows the best available evidence for the management of psoriasis in special locations (e.g., nail psoriasis, palmoplantar psoriasis, psoriasis in skin folds). | (1)60 |
| 24. Pharmacovigilance protocol | The unit has a pharmacovigilance protocol that covers the management and notification of adverse events to the relevant health authorities. | (2)32,55 |
| 25. Protocol for referring patients to rheumatology | The unit has a specific protocol for referring patients with suspected psoriatic arthritis to the rheumatology department; this protocol is designed in collaboration with said department. | (1)41 |
| 26. Hospitalization protocol | The unit has a protocol for the hospitalization of defined patients that specifies which criteria the patients must meet in order to be considered for admission. | Scientific committee |
| 27. Specific protocol for treatment switches | The unit has a specific up-to-date protocol and/or follows the recommendations of national and/or international clinical practice guidelines including the definition of therapeutic failure and specifying the criteria for treatment switches. | (1)56 |
| 28. Protocol for biologic dose adjustments | The unit has a specific up-to-date protocol, designed in collaboration with the hospital pharmacy, and/or follows the recommendations of national and/or international clinical practice guidelines for biologic dose adjustments when considered clinically appropriate. | (1)31 |
| Unit staff quality and organization | ||
| 29. Coordinated collaboration with other health care professionals experienced in psoriasis | The unit works in a coordinated fashion with health care professionals from other areas who are experienced in the management of patients with psoriasis (pharmacists, nursing staff, rheumatologists, etc.). | (5)28,39,41,55,56 |
| 30. Regular interdisciplinary meetings for unit staff | The unit holds multidisciplinary meetings for its staff to update them on the situation of the unit. | (1)55 |
| 31. Identifiable clinical care specialist assigned to each patient | The unit assigns a specific identifiable psoriasis specialist to the care of each patient. | (1)56 |
| 32. Up-to-date training of health care professionals in the unit | The unit guarantees the continuous professional development of its health care staff, including regular training sessions, courses, and other initiatives. | (6)35,41,43,45,55,56 |
| 33. Training of health care professionals external to the unit | The unit provides training to health care professionals who do not form part of but collaborate with the unit, such as rheumatologists, internal medicine specialists, nursing staff, pharmacists, etc. | (3)28,41,55 |
| Admissions to unit: assessments and quality | ||
| 34. Complete diagnosis reflected in the patient's medical record | A note of the following is included in the medical record of all patients in the unit diagnosed with psoriasis: medical history, comorbidities, concomitant medication, previous medication for the management of psoriasis, and results of general physical examination (weight, height, blood pressure, etc.) and physical examination aimed at ruling out psoriatic arthritis. | Scientific committee |
| Patient follow-up: hospitalization | ||
| 35. Participation of dermatologists during patient admission | The health care professionals attached to the unit are available to provide care and follow-up to patients under outpatient follow-up who are admitted to hospital. | (1)56 |
| Patient follow-up: treatment evaluation | ||
| 36. Evaluation of treatment viability prior to initiation of systemic therapy | The unit performs diverse tests (blood tests, biochemical and metabolic analyses, immune status, etc.) and rules out comorbidities or conditions that might interfere with or contraindicate a given treatment before it is prescribed. | Scientific committee |
| 37. Assessment of PASI and DLQI before initiation of systemic therapy | The unit assesses PASI and DLQI scores at the start of any treatment. | (1)59 |
| 38. Monitoring of systemic treatment | The unit has a surveillance program to prevent, detect, and rapidly treat adverse effects associated with systemic treatment. | (1)59 |
| 39. Regular assessment of tolerability and effectiveness of phototherapy | The unit has constant and direct contact with health care professionals responsible for administering phototherapy (including nursing staff) to detect possible problems related to this treatment. | (1)59 |
| Patient follow-up: monitoring | ||
| 40. Regular screening for comorbidities | The unit annually screens for comorbidities associated with psoriasis in patients under treatment with biologics. | (4)33,50,51,60 |
| 41. Monitoring of liver function | The unit performs annual liver function tests in patients on drugs with hepatotoxic potential or patients with liver conditions such as chronic hepatitis virus infection or fatty liver disease. | (3)59–61 |
| 42. Regular monitoring of patients with blood tests | The unit regularly monitors the health of patients on psoriasis treatment via complete blood counts and biochemistry analyses. These tests are performed annually or sooner if changes in PASI, BSA, and/or DLQI are detected. | (1)40 |
| Patient follow-up: management of infection risks | ||
| 43. Updating of immune status of patients scheduled to receive systemic therapy | The unit has a vaccination protocol for patients who are to receive systemic therapy (including biologics); the protocol is designed in conjunction with the internal or preventive medicine department. | (3)46,47,61 |
| 44. Screening for infections | Prior to the initiation of systemic therapy (including biologics), the unit performs the necessary tests to screen for the following microorganisms: HBV, HCV, HIV, and Treponema pallidum. | (2)46,62 |
| 45. Antiviral treatment in HBsAg-positive patients | Prior to the prescription of any systemic or biologic treatment and after checking HBV infection status, the unit refers all patients with signs of infection to the gastroenterology department to receive appropriate antiviral treatment based on criteria agreed on with said department. | Scientific committee |
| 46. Screening for tuberculosis before treatment initiation | The unit screens for tuberculosis before initiating treatment with biologic or systemic drugs. Screening includes tests agreed on with the department of infectious diseases and/or the tuberculosis unit. | (4)43,46,60,62 |
| Patient follow-up: measures for promoting healthy lifestyle habits | ||
| 47. Patient education on healthy lifestyle habits | The unit promotes the adoption of healthy lifestyle habits among patients under treatment. | (3)29,30,34 |
| 48. Smoking cessation program | The unit has a program or referral pathway to help patients stop smoking. | (1)34 |
| 49. Weight loss program | The unit has a program or referral pathway to help patients lose weight. | (1)34 |
| 50. Interventions for psychiatric and psychologic comorbidities | The unit contemplates psychiatric and psychologic comorbidities in patients with psoriasis (anxiety, depression, etc.) and has a program or referral pathway for providing specific help with the management of these comorbidities. | (5)34,49,50,55,64 |
| 51. Assessment of patient lifestyle | The unit assesses the lifestyle of patients with psoriasis to determine the frequency with which they exercise and understand aspects such as diet, alcohol consumption, and smoking habits. | (2)40,51 |
| 52. Training on treatment administration | The unit has staff (dermatology/nursing staff and/or the hospital pharmacist) who instruct patients on how to correctly administer their treatments and record progress in the patients’ medical records. Part of this program targets patients on subcutaneous treatments who are afraid of injections or those on oral treatments who have swallowing difficulties. | Scientific committee |
| Patient follow-up: evaluation of PROMs | ||
| 53. Inclusion of HRQOL as a treatment goal | The unit includes improved HRQOL as a treatment goal for its patients. | (1)42 |
| 54. Evaluation of patient satisfaction with treatment | The unit annually assesses patient satisfaction with treatments they are receiving and records this information in their medical records. | (3)56,60,64 |
| 55. Assessment of impact on patient HRQOL | The unit assesses the impact of psoriasis on patients using the DLQI or other HRQOL tools and records this information in the patients’ medical records. | (2)40,60 |
| 56. Assessment of treatment adherence | The unit regularly assesses levels of treatment adherence among patients with psoriasis. This assessment should be performed by a dermatologist, a nurse, or the hospital pharmacist. | (1)60 |
| Active communication with patients | ||
| 57. Patient health care education | The unit includes patient education in its patient management goals in order to improve their understanding of their disease. Accordingly, the unit provides, on request, patients with educational material in the format best suited to their needs (digital material, diagrams/pictures, scientific articles, etc.). | (11)28,29,36,37,42,46,49,50,52,53,55 |
| 58. Transparency in drug prescription | The unit implements actions aimed at strengthening patient autonomy, providing them with adequate information and allowing them to take informed decisions. Accordingly, it explains the risks and benefits of treatments selected to manage their psoriasis and describes the results they can expect to obtain. To this end, it provides relevant information to the patient in oral and/or written form for improved understanding. The process must be reflected in the patients’ medical records. | (7)29,40,46,54,58,59,63 |
| 59. Shared decision-making and goal setting | The unit takes treatment decisions and establishes treatment goals in a shared process with the patient. The process must be reflected in the patients’ medical records. | (12)29,36–38,40,42,46,48,50,54,58,64 |
| 60. Referrals to patient associations | The unit provides patients with information on patient associations | (2)49,55 |
| 61. Unit contact information | The unit has a system in place to provide patients with the unit's contact information (telephone number and opening hours). | (1)56 |
| Contribution to scientific research | ||
| 62. Research projects | The unit conducts or participates in scientific research projects and contributes to and promotes scientific publications about psoriasis. | (2)55,56 |
| 63. Participation in clinical trials | The unit offers interested patients the option of participating in clinical trials, whether within the unit or in another unit with research facilities. | Scientific committee |
| 64. Participation in working groups/study groups | The unit participates in collaborative studies with other departments and/or psoriasis units. | Delphi panel |
| Outcome indicators | ||
| Clinical performance variables | ||
| 65. Patients being treated at the unit with adequate psoriasis control | More than 50% of patients—only those who have been under follow-up at the unit for at least 1 year and who are receiving systemic treatment or biologic drugs—have a PASI score of 3 or less. | Scientific committee |
| 66. Follow-up of patients at the unit | At least 80% of patients are seen for at least 2 follow-up visits (with the dermatologist and/or nursing staff) at the unit. These visits can be in person or remote (telephone or video call). | Scientific committee |
| 67. Patients from the unit with adequate management of comorbidities associated with psoriasis | Patients referred to a rheumatologist by the dermatologist to screen for suspected psoriatic arthritis should be seen within 3 months at most from time of referral. Seventy percent of patients are seen within this time frame. | Scientific committee |
| PROMs and PREMs | ||
| 68. Patients being treated at the unit with good HRQOL | More than 50% of patients—only those who have under follow-up at the unit for at least 1 year and who are receiving systemic treatment or biologic drugs—have a DLQI score of 5 or less. | Scientific committee |
| 69. Satisfaction with disease course among patients with psoriasis | At least 70% of patients under follow-up in the psoriasis unit report they are satisfied with the course of their disease (score of ≥7 on a scale of 0–10). | Delphi panel |
Abbreviations: AEDV, Spanish Association of Dermatology and Venereology; BSA; body surface area; DLQI, Dermatology Life Quality Index; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HRQOL, health-related quality of life; PASI, Psoriasis Area and Severity Index; PREMs, patient-reported experience measures; PROMs, patient-reported outcome measures.
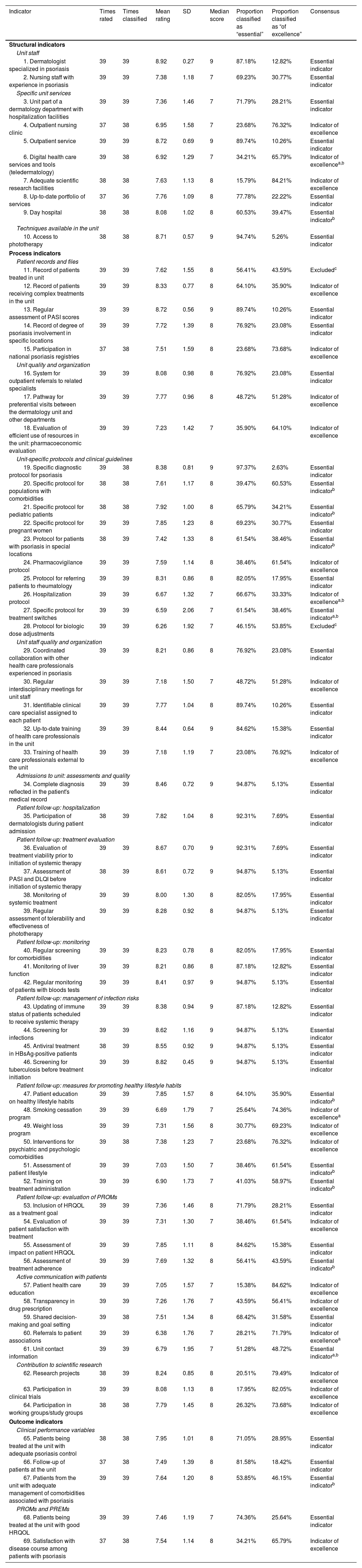
Six indicators (3 structural, 3 process, and 2 outcome) were added after this round, giving a total of 69 indicators to evaluate in round 2 (Table 1). Thirty-nine dermatologists participated in this second round.
The results from the second round and levels of consensus regarding the inclusion and exclusion of indicators and their classification as “essential” or “of excellence” are shown in Table 2.
Results of the Delphi Consensus Process.
| Indicator | Times rated | Times classified | Mean rating | SD | Median score | Proportion classified as “essential” | Proportion classified as “of excellence” | Consensus |
|---|---|---|---|---|---|---|---|---|
| Structural indicators | ||||||||
| Unit staff | ||||||||
| 1. Dermatologist specialized in psoriasis | 39 | 39 | 8.92 | 0.27 | 9 | 87.18% | 12.82% | Essential indicator |
| 2. Nursing staff with experience in psoriasis | 39 | 39 | 7.38 | 1.18 | 7 | 69.23% | 30.77% | Essential indicator |
| Specific unit services | ||||||||
| 3. Unit part of a dermatology department with hospitalization facilities | 39 | 39 | 7.36 | 1.46 | 7 | 71.79% | 28.21% | Essential indicator |
| 4. Outpatient nursing clinic | 37 | 38 | 6.95 | 1.58 | 7 | 23.68% | 76.32% | Indicator of excellence |
| 5. Outpatient service | 39 | 39 | 8.72 | 0.69 | 9 | 89.74% | 10.26% | Essential indicator |
| 6. Digital health care services and tools (teledermatology) | 39 | 38 | 6.92 | 1.29 | 7 | 34.21% | 65.79% | Indicator of excellencea,b |
| 7. Adequate scientific research facilities | 38 | 38 | 7.63 | 1.13 | 8 | 15.79% | 84.21% | Indicator of excellence |
| 8. Up-to-date portfolio of services | 37 | 36 | 7.76 | 1.09 | 8 | 77.78% | 22.22% | Essential indicator |
| 9. Day hospital | 38 | 38 | 8.08 | 1.02 | 8 | 60.53% | 39.47% | Essential indicatorb |
| Techniques available in the unit | ||||||||
| 10. Access to phototherapy | 38 | 38 | 8.71 | 0.57 | 9 | 94.74% | 5.26% | Essential indicator |
| Process indicators | ||||||||
| Patient records and files | ||||||||
| 11. Record of patients treated in unit | 39 | 39 | 7.62 | 1.55 | 8 | 56.41% | 43.59% | Excludedc |
| 12. Record of patients receiving complex treatments in the unit | 39 | 39 | 8.33 | 0.77 | 8 | 64.10% | 35.90% | Indicator of excellence |
| 13. Regular assessment of PASI scores | 39 | 39 | 8.72 | 0.56 | 9 | 89.74% | 10.26% | Essential indicator |
| 14. Record of degree of psoriasis involvement in specific locations | 39 | 39 | 7.72 | 1.39 | 8 | 76.92% | 23.08% | Essential indicator |
| 15. Participation in national psoriasis registries | 37 | 38 | 7.51 | 1.59 | 8 | 23.68% | 73.68% | Indicator of excellence |
| Unit quality and organization | ||||||||
| 16. System for outpatient referrals to related specialists | 39 | 39 | 8.08 | 0.98 | 8 | 76.92% | 23.08% | Essential indicator |
| 17. Pathway for preferential visits between the dermatology unit and other departments | 39 | 39 | 7.77 | 0.96 | 8 | 48.72% | 51.28% | Indicator of excellence |
| 18. Evaluation of efficient use of resources in the unit: pharmacoeconomic evaluation | 39 | 39 | 7.23 | 1.42 | 7 | 35.90% | 64.10% | Indicator of excellence |
| Unit-specific protocols and clinical guidelines | ||||||||
| 19. Specific diagnostic protocol for psoriasis | 39 | 38 | 8.38 | 0.81 | 9 | 97.37% | 2.63% | Essential indicator |
| 20. Specific protocol for populations with comorbidities | 38 | 38 | 7.61 | 1.17 | 8 | 39.47% | 60.53% | Essential indicatorb |
| 21. Specific protocol for pediatric patients | 38 | 38 | 7.92 | 1.00 | 8 | 65.79% | 34.21% | Essential indicatorb |
| 22. Specific protocol for pregnant women | 39 | 39 | 7.85 | 1.23 | 8 | 69.23% | 30.77% | Essential indicator |
| 23. Protocol for patients with psoriasis in special locations | 38 | 39 | 7.42 | 1.33 | 8 | 61.54% | 38.46% | Essential indicatorb |
| 24. Pharmacovigilance protocol | 39 | 39 | 7.59 | 1.14 | 8 | 38.46% | 61.54% | Indicator of excellence |
| 25. Protocol for referring patients to rheumatology | 39 | 39 | 8.31 | 0.86 | 8 | 82.05% | 17.95% | Essential indicator |
| 26. Hospitalization protocol | 39 | 39 | 6.67 | 1.32 | 7 | 66.67% | 33.33% | Indicator of excellencea,b |
| 27. Specific protocol for treatment switches | 39 | 39 | 6.59 | 2.06 | 7 | 61.54% | 38.46% | Essential indicatora,b |
| 28. Protocol for biologic dose adjustments | 39 | 39 | 6.26 | 1.92 | 7 | 46.15% | 53.85% | Excludedc |
| Unit staff quality and organization | ||||||||
| 29. Coordinated collaboration with other health care professionals experienced in psoriasis | 39 | 39 | 8.21 | 0.86 | 8 | 76.92% | 23.08% | Essential indicator |
| 30. Regular interdisciplinary meetings for unit staff | 39 | 39 | 7.18 | 1.50 | 7 | 48.72% | 51.28% | Indicator of excellence |
| 31. Identifiable clinical care specialist assigned to each patient | 39 | 39 | 7.77 | 1.04 | 8 | 89.74% | 10.26% | Essential indicator |
| 32. Up-to-date training of health care professionals in the unit | 39 | 39 | 8.44 | 0.64 | 9 | 84.62% | 15.38% | Essential indicator |
| 33. Training of health care professionals external to the unit | 39 | 39 | 7.18 | 1.19 | 7 | 23.08% | 76.92% | Indicator of excellence |
| Admissions to unit: assessments and quality | ||||||||
| 34. Complete diagnosis reflected in the patient's medical record | 39 | 39 | 8.46 | 0.72 | 9 | 94.87% | 5.13% | Essential indicator |
| Patient follow-up: hospitalization | ||||||||
| 35. Participation of dermatologists during patient admission | 38 | 39 | 7.82 | 1.04 | 8 | 92.31% | 7.69% | Essential indicator |
| Patient follow-up: treatment evaluation | ||||||||
| 36. Evaluation of treatment viability prior to initiation of systemic therapy | 39 | 39 | 8.67 | 0.70 | 9 | 92.31% | 7.69% | Essential indicator |
| 37. Assessment of PASI and DLQI before initiation of systemic therapy | 38 | 39 | 8.61 | 0.72 | 9 | 94.87% | 5.13% | Essential indicator |
| 38. Monitoring of systemic treatment | 39 | 39 | 8.00 | 1.30 | 8 | 82.05% | 17.95% | Essential indicator |
| 39. Regular assessment of tolerability and effectiveness of phototherapy | 39 | 39 | 8.28 | 0.92 | 8 | 94.87% | 5.13% | Essential indicator |
| Patient follow-up: monitoring | ||||||||
| 40. Regular screening for comorbidities | 39 | 39 | 8.23 | 0.78 | 8 | 82.05% | 17.95% | Essential indicator |
| 41. Monitoring of liver function | 39 | 39 | 8.21 | 0.86 | 8 | 87.18% | 12.82% | Essential indicator |
| 42. Regular monitoring of patients with bloods tests | 39 | 39 | 8.41 | 0.97 | 9 | 94.87% | 5.13% | Essential indicator |
| Patient follow-up: management of infection risks | ||||||||
| 43. Updating of immune status of patients scheduled to receive systemic therapy | 39 | 39 | 8.38 | 0.94 | 9 | 87.18% | 12.82% | Essential indicator |
| 44. Screening for infections | 39 | 39 | 8.62 | 1.16 | 9 | 94.87% | 5.13% | Essential indicator |
| 45. Antiviral treatment in HBsAg-positive patients | 38 | 39 | 8.55 | 0.92 | 9 | 94.87% | 5.13% | Essential indicator |
| 46. Screening for tuberculosis before treatment initiation | 39 | 39 | 8.82 | 0.45 | 9 | 94.87% | 5.13% | Essential indicator |
| Patient follow-up: measures for promoting healthy lifestyle habits | ||||||||
| 47. Patient education on healthy lifestyle habits | 39 | 39 | 7.85 | 1.57 | 8 | 64.10% | 35.90% | Essential indicatorb |
| 48. Smoking cessation program | 39 | 39 | 6.69 | 1.79 | 7 | 25.64% | 74.36% | Indicator of excellencea |
| 49. Weight loss program | 39 | 39 | 7.31 | 1.56 | 8 | 30.77% | 69.23% | Indicator of excellence |
| 50. Interventions for psychiatric and psychologic comorbidities | 39 | 38 | 7.38 | 1.23 | 7 | 23.68% | 76.32% | Indicator of excellence |
| 51. Assessment of patient lifestyle | 39 | 39 | 7.03 | 1.50 | 7 | 38.46% | 61.54% | Essential indicatorb |
| 52. Training on treatment administration | 39 | 39 | 6.90 | 1.73 | 7 | 41.03% | 58.97% | Essential indicatorb |
| Patient follow-up: evaluation of PROMs | ||||||||
| 53. Inclusion of HRQOL as a treatment goal | 39 | 39 | 7.36 | 1.46 | 8 | 71.79% | 28.21% | Essential indicator |
| 54. Evaluation of patient satisfaction with treatment | 39 | 39 | 7.31 | 1.30 | 7 | 38.46% | 61.54% | Indicator of excellence |
| 55. Assessment of impact on patient HRQOL | 39 | 39 | 7.85 | 1.11 | 8 | 84.62% | 15.38% | Essential indicator |
| 56. Assessment of treatment adherence | 39 | 39 | 7.69 | 1.32 | 8 | 56.41% | 43.59% | Essential indicatorb |
| Active communication with patients | ||||||||
| 57. Patient health care education | 39 | 39 | 7.05 | 1.57 | 7 | 15.38% | 84.62% | Indicator of excellence |
| 58. Transparency in drug prescription | 39 | 39 | 7.26 | 1.76 | 7 | 43.59% | 56.41% | Indicator of excellence |
| 59. Shared decision-making and goal setting | 39 | 38 | 7.51 | 1.34 | 8 | 68.42% | 31.58% | Essential indicator |
| 60. Referrals to patient associations | 39 | 39 | 6.38 | 1.76 | 7 | 28.21% | 71.79% | Indicator of excellencea |
| 61. Unit contact information | 39 | 39 | 6.79 | 1.95 | 7 | 51.28% | 48.72% | Essential indicatora,b |
| Contribution to scientific research | ||||||||
| 62. Research projects | 38 | 39 | 8.24 | 0.85 | 8 | 20.51% | 79.49% | Indicator of excellence |
| 63. Participation in clinical trials | 39 | 39 | 8.08 | 1.13 | 8 | 17.95% | 82.05% | Indicator of excellence |
| 64. Participation in working groups/study groups | 38 | 38 | 7.79 | 1.45 | 8 | 26.32% | 73.68% | Indicator of excellence |
| Outcome indicators | ||||||||
| Clinical performance variables | ||||||||
| 65. Patients being treated at the unit with adequate psoriasis control | 38 | 38 | 7.95 | 1.01 | 8 | 71.05% | 28.95% | Essential indicator |
| 66. Follow-up of patients at the unit | 37 | 38 | 7.49 | 1.39 | 8 | 81.58% | 18.42% | Essential indicator |
| 67. Patients from the unit with adequate management of comorbidities associated with psoriasis | 39 | 39 | 7.64 | 1.20 | 8 | 53.85% | 46.15% | Essential indicatorb |
| PROMs and PREMs | ||||||||
| 68. Patients being treated at the unit with good HRQOL | 39 | 39 | 7.46 | 1.19 | 7 | 74.36% | 25.64% | Essential indicator |
| 69. Satisfaction with disease course among patients with psoriasis | 37 | 38 | 7.54 | 1.14 | 8 | 34.21% | 65.79% | Indicator of excellence |
Abbreviations: DLQI, Dermatology Life Quality Index; HBsAg, hepatitis B surface antigen; HRQOL, health-related quality of life; PASI, Psoriasis Area and Severity Index; PREMs, patient-reported experience measures; PROMs, patient-reported outcome measures.
The final set comprised 67 indicators (10 for structure, 52 for process, and 5 for outcomes); 45 were classified as “essential” and 22 as “of excellence”.
DiscussionSpain has highly qualified, committed professionals who provide care to patients with psoriasis in specialized units. There is still, however, potential room for improvement in clinical practice through standardization of care across units and autonomous regions and continued quality improvement of services.
The AEDV's Psoriasis Group is a leading authority on training and research in the field of psoriasis. Its participation in the CUDERMA project is therefore crucial for driving further improvements and guaranteeing quality in the provision of care to patients with psoriasis.
The aim of this first phase of the CUDERMA project targeting psoriasis units was to apply the Delphi technique to identify and achieve consensus on criteria that would define a unit meeting the basic conditions and standards required to provide adequate treatment and follow-up care to patients; a second aim was to identify criteria that define excellence. The process resulted in the first set of quality indicators for use in the certification of specialized psoriasis units.
Aspects identified by the panelists as “essential” were classified into the following subcategories: 1) dermatologists and nurses trained in the latest advances in psoriasis; 2) access to adequate facilities, techniques, and therapies; 3) patient registries and recording of patient information, diagnoses, and other variables relevant to psoriasis; 4) contact with other specialists involved in the management of psoriasis; 5) protocols and strategies for promoting treatment effectiveness and safety (including training on treatment administration); 6) monitoring of comorbidities; 7) health education; 8) inclusion of patient-reported outcome measures; and 9) joint physician–patient decision-making.
Quality indicators classified as “of excellence” were divided into 12 subcategories: 1) presence of an outpatient nursing clinic; 2) access to digital tools; 3) scientific research; 4) registries of patients with complex treatments and participation in national registries; 5) pathways for preferential visits; 6) evaluation of efficient use of resources; 7) pharmacovigilance and hospitalization protocols; 8) regular multidisciplinary meetings; 9) training of health care professionals not directly attached to the unit; 10) promotion of healthy lifestyle habits and mental health; 11) evaluation of patient-reported experiences; and 12) patient empowerment.
One of the strengths of this study is the distinction it makes between indicators that measure aspects considered to be essential and those considered to add value, as this means that units with different characteristics and resources can seek certification. It also provides hospitals with more experience and higher levels of activity with the means to aspire to excellence and promote continuous improvements in quality of care provision.
Another strength of the CUDERMA project is the use of a multidisciplinary group to evaluate the preliminary set of indicators, as incorporating the perspectives of other specialists involved in the care of patients with psoriasis provides the indicators with a broader scope.
Although involvement of the multidisciplinary group formed by nondermatology specialists and patients is a strength of the study, it should be noted that the members of this group only participated in the initial phase (i.e., they did not participate in the Delphi consensus process). Particular efforts were thus made to preserve their original contributions.
Finally, the CUDERMA project differs from other consensus-based studies that have developed quality indicators in that it has 2 separate stages: one to agree on which aspects should be measured and another to define the resulting quality indicators (name, definition, standard, objective criteria for compliance, and evidence of compliance). The selected indicators will therefore be standardized for subsequent certification of units, demonstrating their relevance for guaranteeing quality of care in psoriasis.
ConclusionsThe first phase of the CUDERMA project targeting psoriasis units has identified a list of aspects that should be evaluated by quality indicators used to certify these units. The consensus process produced 67 indicators: 10 structural indicators, 52 process indicators, and 5 outcome indicators. Forty-five were classified as “essential” and 22 as “of excellence”. The quality indicators will be standardized in subsequent phases of the CUDERMA project to produce a definitive set for certifying psoriasis units.
FundingThe CUDERMA project is an initiative of the AEDV and is funded by an unrestricted grant from Abbvie.
Conflicts of InterestA. de la Cuadra-Grande is an employee at Pharmacoeconomics & Outcomes Research Iberia (PORIB), a consultancy firm specialized in the economic evaluation of health interventions and health outcome research; he has received payment for methodological support throughout the project from AEDV. The other authors declare no conflicts of interest.
The authors would like to thank Miguel Ángel Casado and Araceli Casado-Gómez, employees of Pharmacoeconomics & Outcomes Research Iberia (PORIB), for their invaluable collaboration in this project.
Cardiometabolic specialist: Alonso, Nuria [Hospital Universitari Germans Trias i Pujol]. Nursing: Castro, Laura [Complejo Hospitalario Universidad de Pontevedra]; de la Torre, Jenny [Hospital General Universitario de Alicante]. Hospital pharmacist: Cardona, Gloria [Hospital Universitari Germans Trias i Pujol]. Family medicine specialist: Cabrerizo, Ana María [Centro de Salud Padul]. Preventive medicine specialist: Valero, María Carmen [Hospital Universitario San Cecilio de Granada]. Patients: Lorenzo, Noela; Rodríguez, Fátima. Rheumatologists: Joven, Beatriz [Hospital Universitario 12 de Octubre]; Queiro, Rubén [Hospital Universitario Central de Asturias].
Abalde, María Teresa [Complejo Hospitalario Universitario de Pontevedra]; Andrés, Juan José [Hospital Vega Baja]; Ara, Mariano [Hospital Clínico Universitario Lozano Blesa]; Armesto, Susana [Hospital Universitario Marqués de Valdecilla]; Aparicio, Gloria [Hospital Universitario Vall d’Hebron]; Beniandrés, Ofelia [Hospital General Universitario Gregorio Marañón]; Carretero, Gregorio [Hospital Universitario de Gran Canaria Doctor Negrín]; Conde-Taboada, Alberto [Hospital Clínico San Carlos]; Ferrán, Marta [Hospital del Mar]; Ferrándiz, Carlos [Instituto Médico Ferrándiz-Pulido]; Galán, Manuel [Hospital Universitario Reina Sofía]; Eiris-Salvado, Noemí [Hospital Universitario Virgen de la Macarena]; García-Bustinduy, Marta [Complejo Hospital Universitario de Canarias en La Laguna]; García-Latasa, Francisco Javier [Hospital Royo Villanova]; García-Patos, Vicente [Hospital Universitario Vall d’Hebron]; González, Alicia [Hospital Universitario de Gran Canaria Doctor Negrín]; Herranz, Pedro [Hospital Universitario La Paz]; Llamas, Mar [Hospital Universitario de la Princesa]; López, Anna [Hospital de la Santa Creu i Sant Pau]; Marrón, Servando Eugenio [Hospital Universitario Miguel Servet]; Martínez, Elena [Hospital Universitario de Toledo]; Martorell, Antonio [Hospital de Manises]; Mataix, Javier [Hospital Marina Baixa]; Mateu, Almudena [Hospital Universitario Doctor Peset]; Pérez, Silvia [Hospital Universitario Basurto]; Puig, Luis [Hospital de la Santa Creu i Sant Pau]; Pujol, Conrado [Hospital la Fe de Valencia]; Romero, Alberto [Hospital Universitario de Fuenlabrada]; Roncero, Mónica [Complejo Asistencial Universitario de Salamanca]; Ruiz, Diana [Hospital Universitario Fundación Alcorcón]; Ruiz-Carrascosa, José Carlos [Hospital Clínico Universitario San Cecilio]; Salleras, Montserrat [Hospital Universitari Sagrat Cor]; Sánchez-Regaña, Manuel [Clínica Dermacot]; Vicente, Asunción [Hospital Sant Joan de Déu]; Zulaica, Ander [Hospital do Meixoeiro].