Cutaneous adverse effects of interferon are common, including particularly injection site reactions, alopecia, stomatitis, pruritus, and flare-ups of psoriasis, eczema, or lichen planus.1 Interferon-induced sarcoidosis is a lesser-known adverse effect that may affect 0.2% of patients.2 The first case of this condition was reported in 1987 in a patient receiving interferon for renal carcinoma3 and the first case secondary to treatment for hepatitis C was published in 1993.4 Since then many cases of interferon-induced sarcoidosis have been reported.2
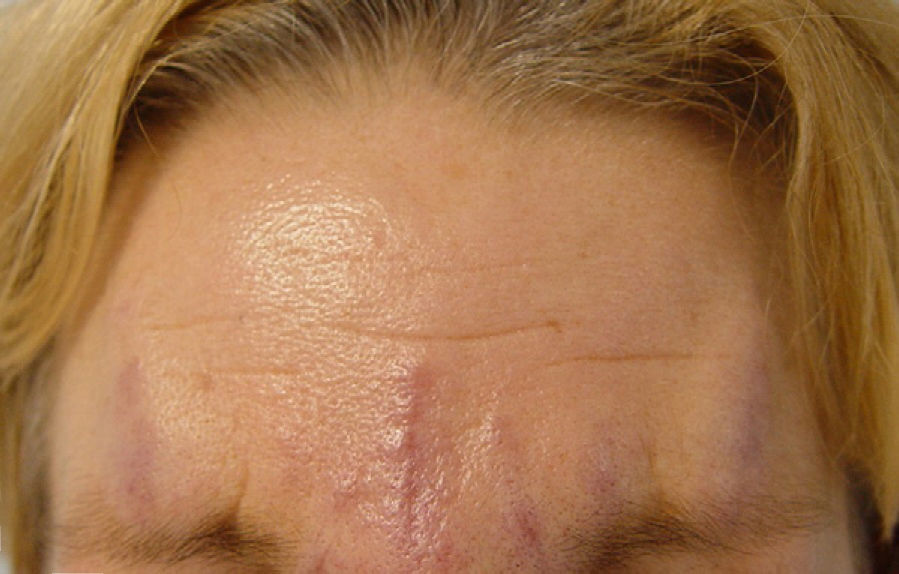
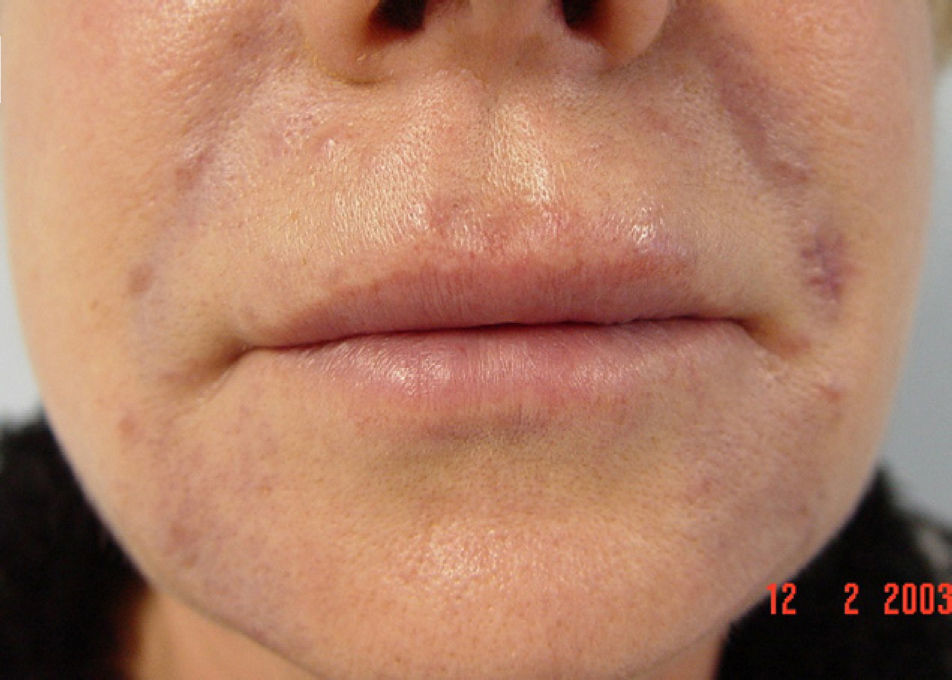
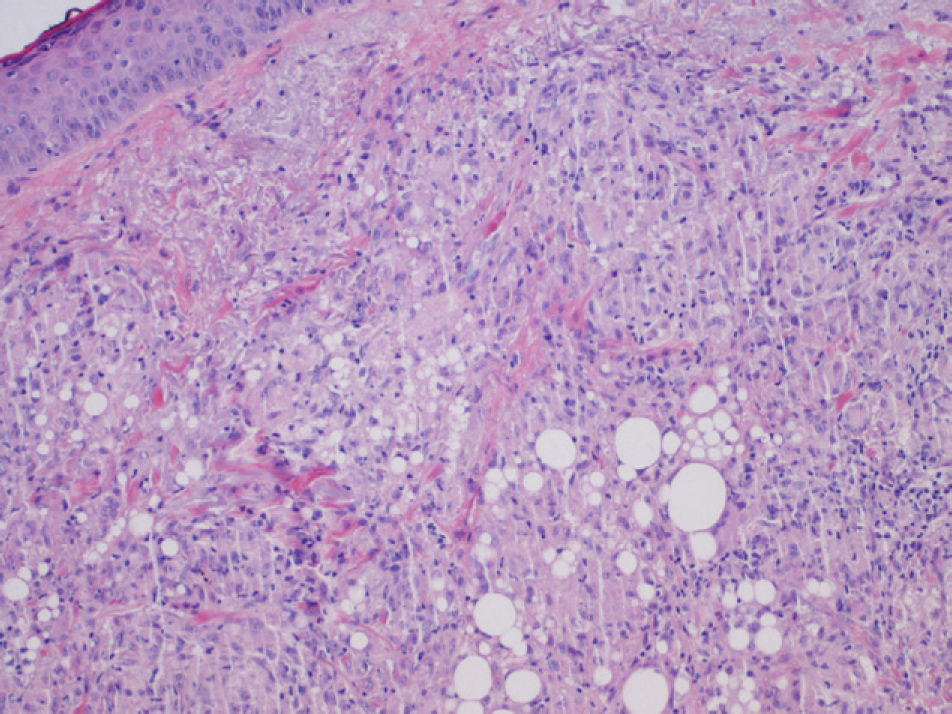
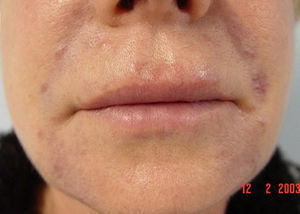
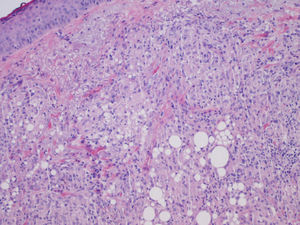
We report the case of a 56-year-old woman who presented with facial lesions that had appeared a month earlier. The patient's history included hepatitis C diagnosed in 2002, for which she had started treatment with pegylated interferon alfa-2b and ribavirin in October 2003. Three months after starting treatment she developed progressive facial edema, so the treatment was discontinued. During the following month the edema gradually remitted and violaceous nodular lesions appeared in the nasolabial folds and on the upper lip and forehead, coinciding with the areas injected with cosmetic filler material, including Artecoll (polymethylmethacrylate microspheres suspended in a 3.5% solution of collagen) 14 and 4 years earlier (Figs. 1 and 2). Skin biopsy revealed in the dermis the presence of epithelioid granulomas with multinucleated giant cells surrounding optically empty vacuolar structures of various sizes (Fig. 3).The following relevant results were observed in the blood tests: aspartate aminotransferase, 60 U/L; alanine aminotransferase, 79 U/L; angiotensin converting enzyme, 145 U/L (normal range, 0-115 U/L). A Mantoux test was negative and a chest radiograph normal. In the absence of other systemic symptoms, no more tests were performed. We established the diagnosis of sarcoidosis induced by interferon and ribavirin, with granulomas in the areas injected with filler material. The patient decided to discontinue the antiviral treatment definitively and the lesions regressed spontaneously in 6 months.
Our first approach was to determine whether the patient was suffering from foreign body granulomas or sarcoidosis lesions, because traditionally the presence of foreign material in epithelioid granulomas excludes the latter diagnosis. However, it is now thought that in sarcoidosis the immune system's ability to eliminate foreign material is altered, so the foreign material acts as a focus for the location of granulomas.5
Although the patient was told that the filler material was Artecoll, the presence of vacuoles of various sizes in the biopsy suggests the presence of silicone, because the granulomas induced by Artecoll usually have vacuoles of a more uniform size.6
Although the first reported case of sarcoidosis associated with hepatitis C occurred during treatment with interferon,4 this condition has also been reported in untreated individuals, so it is postulated that hepatitis C could also favor its development. In a study of 68 patients with hepatitis C and sarcoidosis by Ramos-Casals et al,2 antiviral treatment proved to be the trigger in 75% of cases. With regard to the pathogenic mechanism, there is a predominantly T helper 1 (Th1) response in sarcoidosis that is very active against a variety of exogenous antigens or autoantigens. By favoring the differentiation of T helper cells towards the Th1 type, interferon could induce the onset of the disease. In addition, ribavirin may act as a cofactor because it also stimulates the Th1 response.
Interferon-induced sarcoidosis is more common in middle-aged women, usually appearing during the first 6 months of treatment. It mainly affects the lungs and skin. Skin lesions have been reported in 60% of the patients, sometimes around foreign bodies7 or on filler material.8,9 The prognosis is generally good, with spontaneous improvement after cessation of treatment.
In the literature review we found 4 cases of sarcoidosis with granulomas in areas injected with filler materials.8–10 All patients were women: one had pulmonary sarcoidosis10 and in the other 3 the disease appeared after interferon and ribavirin treatment for hepatitis C.8,9 The fillers were hyaluronic acid,9 silicone,9 and Artecoll8,10 and they had been injected 2 to 10 years earlier. In the 3 cases induced by interferon, treatment was not discontinued: in 2 cases oral corticosteroids were administered9 and in 1 case allopurinol8; the lesions improved in all 3 cases. Therefore, the therapeutic approach to interferon-induced sarcoidosis depends on the severity of the disease. If there is no significant systemic involvement, the therapy can be maintained with suitable follow-up and treatment.2
In conclusion, because the use of cosmetic filler materials is growing, it is likely that more cases like ours will be diagnosed in the future. We suggest requesting hepatitis C serology, asking the patients about a possible past history of sarcoidosis, and warning of this potential adverse effect prior to injection with filler material.
Please cite this article as: López-Pestaña A, et al. Granulomas sarcoideos en material de relleno facial inducidos por interferon α y ribavirina en pacientes con hepatitis C. Actas Dermosifiliogr.2011;102:746-747.