Rituximab was introduced into clinical practice as a medication with considerable potential. Its use in patients with B-cell lymphoma and rheumatoid arthritis revealed numerous indications in autoimmune diseases, many of which involve the skin, thus requiring dermatologists to become familiar with both the characteristics of anti-CD20 antibodies and the role of B cells in multiple skin diseases. Thanks to these developments, we will be able to use rituximab more frequently and appropriately in our patients and draw up consensus guidelines based on large case series. In other words, establishing the indications for rituximab will make it possible to shorten disease course and reduce morbidity due to more specific drugs.
El rituximab se ha introducido en Medicina como un agente terapéutico con un futuro muy prometedor. Después de su empleo en casos de linfomas B y artritis reumatoide, son numerosas las indicaciones que se han establecido dentro de las enfermedades autoinmunes, muchas de ellas dermatológicas. Es por ello, que los dermatólogos debemos familiarizarnos con las características de los anticuerpos anti-CD20, así como con el papel de las células B en muchas enfermedades cutáneas. Estos dos hechos, permitirán que pueda utilizarse este fármaco cada vez más y mejor en nuestros pacientes, y a su vez puedan establecerse guías consensuadas de su uso basadas en series amplias de pacientes. Es decir, podremos establecer en qué circunstancias y situaciones Rtx estará indicado, acortando de esta manera la enfermedad y disminuyendo la morbilidad en los enfermos por el empleo de fármacos más específicos.
The introduction of new therapies has marked a watershed in in the treatment of diseases in most specialties, dermatology included. In some cases, an existing drug has been used in a novel off-label indication while in others a new agent has been used for the first time. Examples include retinoids for the treatment of psoriasis or acne,1,2 ciclosporin, which was initially indicated as an immunosuppressant in transplantations and then subsequently used in a range of autoimmune skin diseases,3 biologic agents in the management of refractory psoriasis,4 and more recently propanolol in the treatment of angiomas,5 to mention but a few. A further example of a therapeutic breakthrough is the use of anti-CD20 antibodies, initially indicated for the treatment of lymphoma,6 and subsequently for autoimmune diseases, many of which are dermatologic. The use of anti-CD20 antibodies in dermatologic conditions is also another example of how management of skin diseases has been influenced by developments in the treatment of oncologic and hematologic conditions. Only when the mechanism of action of new agents has been understood have they been used in other diseases.
Most dermatologists are familiar with the indications approved by the US Food and Drug Administration (FDA) for anti-CD20 antibodies (rituximab). However, rituximab is playing an important role in the treatment and management of an increasing number of other conditions, mainly because, contrary to past thinking, the agent is useful not only in indications in which autoantibodies play an important role but also in diseases in which B cells are implicated in the pathogenesis. A key consequence of this change in thinking about rituximab is that our knowledge of the role of B cells in autoimmunity has been extended,6 giving rise to the concept of B-cell directed therapy for inflammatory skin diseases7 and defining the significance and therapeutic scope of the drug. The discovery that the function and role of B cells in autoimmune diseases is broader than previously thought has led to the use of rituximab in certain B-cell mediated diseases regardless of whether autoantibodies play a role.
Several concepts follow from the above insight and will form the basis of this review of rituximab in dermatology. Specifically, we will discuss the indications, characteristics, and mechanism of action of this agent, as well as issues that should be taken into account when treating patients with rituximab.
Drug CharacteristicsRituximab is a chimeric immunoglobulin (Ig) G1 κ monoclonal antibody composed of a murine fragment, antibody, binding (Fab) region, and a human fragment crystallizable (Fc) region.8–10 It binds specifically to a transmembrane protein, the CD20 antigen, which is expressed on B cells (from pre-B cells through to preplasma cells), but not on the least mature lymphopoietic cells or on mature plasma cells. The selectivity of rituximab for cells that express this antigen keeps the impact on Ig production to a minimum and enables stem cells to reset B-cell production at some time after therapy has finished. In other words and as discussed in more depth later, although the antibody-producing cells are not directly eliminated, rituximab depletes memory B cells (precursors of the CD20− plasma cells) thereby indirectly inhibiting autoantibody production for a prolonged period.The function of the CD20 antigen and its actual ligand are not known,11 although we do know though that the action of rituximab in CD20+ cells varies according to the type of cell. Thus, for example, anti-CD20 antibodies act more selectively on circulating cells and on cells in the follicular region of the spleen than on cells in the marginal zone of the lymph node.12–14
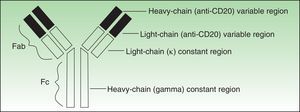
Antibodies used to treat patients can be of various types: human (the entire molecule has the structure observed in humans), humanized (the constant region is entirely human, as is part of the variable region; thus almost the entire antibody is human), murine or derived from other animal species (the entire molecule is a copy of another animal species), or chimeric (the constant region is fully human while the variable region is derived from another species) (Fig. 1).
Although rituximab has been used for many years and its efficacy demonstrated since 1987,1 the FDA did not approve the agent as first-line treatment in advanced follicular lymphoma until 2006. The approval for rheumatoid arthritis was granted in the same year. The indications have since expanded to other lymphoproliferative processes such as diffuse large B-cell lymphoma and chronic lymphocytic leukemia. Currently, there is no officially approved indication for rituximab in dermatologic processes, although the agent is included in some consensus statements as a treatment option for certain skin diseases when conventional treatment has not been effective.9,15
Mechanism of Action of RituximabTo understand the mechanism of action of rituximab, we must first understand the role of B cells in autoimmune diseases. In fact, we now know more about many of the actions of these cells in such conditions thanks to the introduction of anti-CD20 antibodies.
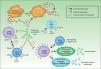
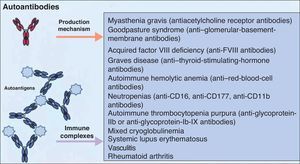
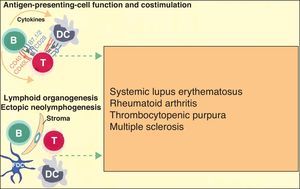
It is accepted that B cells contribute to body function and play a part in many diseases through antibody or autoantibody production. In fact, for years, it was thought that this was almost the only relevant action of these cells. Most authors accepted that the presence of autoantibodies was an essential requisite for assigning a role to B cells in autoimmune diseases. The presence of such autoantibodies was included in diagnostic and prognostic criteria, and they were considered to play a key role in the pathogenesis of certain diseases. Thus, autoantibodies were implicated in processes in which their action, through binding to specific antigens, produced a given disease. Measurement of these autoantibodies in peripheral blood facilitated the diagnosis of the disease and was an important prognostic factor. It was shown that many of these autoantibodies enhance tissue damage by binding of their Fc region to inflammatory cells that further increased local inflammation. B cells were also found to be implicated in the pathogenesis of other diseases through the production of autoantibodies and the formation of circulating immune complexes. These antibodies were thought to be responsible for tissue damage in the tissues where they accumulate. This phenomenon has been described in conditions such as cryoglobulin-induced leukocytoclastic vasculitis and lupus nephritis (Fig. 2).6 The detection of polymorphisms in high- and low-affinity Fc receptors in certain diseases, such as systemic lupus erythematosus (SLE) and thrombocytopenic purpura, may be a marker of specificity in the immune response.16
Autoantibodies produced by B cells can mediate tissue damage through binding to specific antigens or by deposition in the tissues once circulating immune complexes have formed. Adapted from Martin F et al.6
More recently, in autoimmune diseases such as SLE, rheumatoid arthritis, and autoimmune thrombocytopenic purpura, it has been reported that B cells may have an additional proinflammatory effect because they act as antigen-presenting cells for T cells, thereby increasing inflammation in tissues through cytokine production and favoring the formation of ectopic lymphoid tissue (Fig. 3).6 The discovery that B cells have a wider range of action than originally described has led to an extension of the indications for B-cell depleting drugs.
B cells can also actively contribute to inflammation in autoimmune diseases through the action of T-cell antigen presenting cells and by costimulating the inflammatory process through the production of cytokines in the medium itself. In addition, they can participate in the inflammatory response through the formation of ectopic lymphoid tissue. Adapted from Martin F et al.6
There are 3 main types of mechanism by which anti-CD20 antibodies act on and ultimately destroy or eliminate CD20+ cells: cell-mediated cytotoxicity triggered by the antibody itself, complement-mediated cell damage, and induction of apoptosis mechanisms in the cells on which they act.17,18
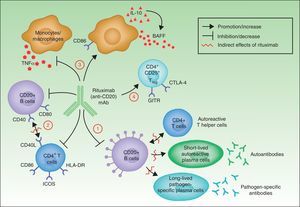
In summary, although the action of rituximab is derived largely from its effects on B cells, its indication in autoimmune diseases is not based solely on its impact on autoantibody production.18 Rituximab has other targets through which it can exhibit its therapeutic effect (Fig. 4).
- 1.
The most widely known benefit of anti-CD20 antibodies, as mentioned earlier, occurs through depletion of CD20+ B cells, leading to a decrease in both the de novo generation of (autoreactive) plasma cells and the production of autoantibodies.
- 2.
Rituximab can also interfere with the expression of CD40 and CD80 on the surface of B cells,19,20 and with that of CD69, inducible costimulator, human leukocyte antigen DR, and CD40L on CD4+ T-helper cells.20–22 Thus, the drug could regulate key costimulation signals, for example, through the CD40-CD40L pair. Such signals are necessary for interaction between B and T cells, and interference in signaling may abrogate the action of these cells in autoreactive immune processes.23
- 3.
Another noteworthy action of rituximab is its effect on the production of certain proinflammatory cytokines. Specifically, it has been found that the drug decreases TNF-α production by monocytes and macrophages, whereas production of interleukin-10 and B-cell activating factor (BAFF) is increased. Interleukin-10 may also increase BAFF levels through autocrine and paracrine mechanisms. Likewise, the expression of CD86 on the surface of monocytes and macrophages is increased.24
- 4.
Finally, anti-CD20 antibodies are thought to act through induction of T regulator CD4+CD25+ cells (Treg). These cells can be identified by increased expression of mRNA for forkhead box P3 (Foxp3), glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) and cytotoxic T-lymphocyte antigen-4 (CTLA-4).25–27
Mechanisms of action of rituximab. Adapted from Nagel A et al.18
All the studies cited above point to the same basic idea: the action of rituximab is broader than first thought, and includes direct action against the production of autoantibodies, intermediate processes in autoimmune response (impact on the B/T cell ratio), and regulation of the immune response itself through an increase in cells that control response. It is also important to point out that the intensity of each of these actions may vary greatly depending on the characteristics of the anti-CD20 antibody under study.
Rituximab produces an effect rapidly but response varies from patient to patient and depending on the disease studied. In rheumatoid arthritis, only 20% of patients had complete B-cell depletion in the synovium at 1 month after treatment,28 although there was an overall decrease in inflammation in all patients. In patients with pemphigus vulgaris, investigators have found a rapid depletion of CD19+ cells (B cells), apparent within days of starting treatment and sustained for between 6 to 9 months on average and even as long as 15 months.29 We too have observed such an effect.30 But the interesting aspect of the effect achieved by this drug is that when the CD20+ cells reappear months later in the bloodstream they are derived from naive cells and have a new profile of antibody production; that is, many of these CD20+ cells have been reset and are not programmed for autoantibody production.28,31,32 The duration of response in each patient depends on the extent to which this resetting occurs.
Dose and AdministrationCurrently, there are 3 rituximab protocols in use in different diseases18:
- 1.
Weekly administration of 375mg/m2 for 4-8 weeks. This regimen was initially used in patients with lymphoma and also in other autoimmune diseases. In 1994, this dose was established as the most effective.33
- 2.
Administration of 2 doses, each of 1000mg, 2 weeks apart. This regimen was initially used in patients with rheumatoid arthritis,34 and subsequently in patients with SLE,35 dermatomyositis,36 or atopic dermatitis.37
- 3.
Finally, anti-CD20 antibodies have also been administered intralesionally, mainly in cutaneous lymphomas (dose of 10mg/mL, with 1mL per lesion administered in several sessions),38 orbital lymphomas,39 and lymphoid hyperplasia.40
Rituximab has also been used in combination with other drugs in some diseases. The therapeutic regimens have changed as knowledge of the action of the drug has improved. In the case of SLE, for example, a single dose of 100mg/m2 or 375mg/m2 yielded similar results to a dose of 375mg/m2 administered at weekly intervals for 4 weeks.41 In patients with rheumatoid arthritis, no additional benefit was observed when a rituximab dose of 375mg/m2 was administered for 4 weekly sessions as compared to 2 sessions.42 In general, treatment with repeated courses of rituximab can improve the patient's condition without increasing the rate of adverse effects.43 There are few large studies in autoimmune blistering diseases; the results obtained in some of these series suggest that just 2 cycles of rituximab can induce a complete response,44 while in others maintenance therapy over longer periods was required to induce response.45 Therefore, we need a more complete understanding of the characteristics of the drug to allow us to design more effective treatment regimens that can be tailored to the target disease and will offer a greater benefit to the patients.
In the scientific literature, several studies evaluate the response to rituximab and other immunomodulator or immunosuppressive agents in autoimmune blistering diseases.9 In general, none of these studies confirm that it is necessary to combine rituximab with other drugs (apart from corticosteroids) to achieve the desired effect. Recently, we studied 28 patients with pemphigus vulgaris and pemphigus foliaceus treated with rituximab according to different regimens (with and without immunoglobulins) and we found that administration of intermediate doses of corticosteroids, along with rituximab 375 mg/m2 for 4 weekly sessions was sufficient to control the disease. Combination with other drugs did not provide any therapeutic benefit to the patients.46
Finally, given the characteristics of the drug, it is important to bear in mind a series of recommendations when administering rituximab, regardless of the disease to be treated. As it is a chimeric murine antibody, this agent should not be administered to patients with known sensitivity to murine proteins. To avoid possible adverse reactions (described later), patients should be premedicated with methylprednisolone, acetaminophen, and diphenhydramine. As a general rule, the rate of the initial infusion should be 50mg/h. If there are no adverse reactions, the infusion rate can be increased by 50mg/h every 30minutes up to a maximum of 400mg/h. If the first infusion is well tolerated, subsequent infusions can start at 100mg/h and be increased to a maximum infusion rate of 400mg/h (see the Summary of Product Characteristics http://www.linfoma.roche.es/FichaTecnicaMabth.pdf).
Adverse ReactionsAdverse reactions to rituximab can be classified into several categories, depending on the type of reaction and the mechanism involved (for more detailed information, see the Summary of Product Characteristics http://www.linfoma.roche.es/FichaTecnicaMabth.pdf).
- 1.
Infusion-related reactions. According to the studies performed, infusion-related reactions appear in more than half the treated patients, usually in the first 2hours of the first infusion. These adverse effects are usually mild (National Cancer Institute [NCI] toxicity grade 1 or 2 in 80% to 90% of the cases),47 and consist mainly of fever, chills, and rigors. Other symptoms included flushing, angioedema, bronchospasm, vomiting, nausea, urticaria/rash, fatigue, headache, throat irritation, rhinitis, pruritus, tachycardia, hypertension, hypotension, dyspnea, and dyspepsia. Severe infusion-related reactions (bronchospasm or hypotension) may occur in up to 10% of patients. Of particular note are the infusion-related cardiac complications. Since pre-existing conditions, such as myocardial infarction, atrial fibrillation, pulmonary edema, and congestive heart failure, are often reactivated by treatment, rituximab is contraindicated in patients with a history of severe cardiac disease. Furthermore, given that hypotension may occur during treatment with rituximab, the protocol recommends the omission of any antihypertensive medication for 12hours before an infusion. The mildest infusion-related reactions generally resolve on reducing the infusion rate. The rate can be increased once again when symptoms improve.
- 2.
Tumor lysis syndrome/cytokine release syndrome. Tumor lysis syndrome and cytokine release syndrome are closely related phenomena that occur mainly in patients with high tumor burden. On destruction of the tumor, large numbers of cytokines are released into the bloodstream. These syndromes are characterized by the presence of generalized urticaria with angioedema associated with fever, bronchospasm, and hypoxia. Hyperuricemia, hyperpotassemia, hypocalcemia, hyperphosphatemia, acute renal failure, and elevated lactate dehydrogenase hormone (LDH) may also be present. Respiratory failure may occur in association with interstitial infiltrate or pulmonary edema. These complications can present in the first or second hour of the first drug infusion. Patients with pulmonary insufficiency or pulmonary tumor infiltration are at a greater risk of developing tumor lysis syndrome; in such cases special precaution should be taken and rituximab administered more slowly. If these side effects are severe, infusion of rituximab should be suspended. If continuation of treatment is possible, it is of interest to know that cytokine release syndrome does not usually recur in subsequent infusions.
- 3.
Hypersensitivity reactions. Hypersensitivity reactions present during the first few minutes of infusion, and are usually anaphylactic. They may resemble cytokine release reactions, but the timing of onset is different. Drugs such as adrenaline, antihistamines, and glucocorticosteroids should be available for immediate treatment of these types of reactions.
- 4.
Infections. The management of infections associated with rituximab is a widely debated topic. On the one hand, this agent is contraindicated in patients with severe active infections (tuberculosis, sepsis, opportunist infections, etc.) and in patients with severe immunosuppression (very low CD4 and CD8 levels). Immunoglobulin levels should be measured before starting treatment with anti-CD20 agents as the risk of infection increases in the presence of hypogammaglobulinemia. The risk is also higher in the presence of neutropenia, which is another side effect that can have a late onset in patients treated with rituximab. The treating physician should therefore be particularly careful when prescribing anti-CD20 agents to patients with chronic or recurrent infections, or when the patient's clinical condition predisposes them to a greater risk of infection. Cases of hepatitis B virus reactivation have been reported so viral load should be measured prior to starting treatment in patients with a history of hepatitis.
Physicians should review the patient's vaccination history and observe current vaccination guidelines before starting treatment with rituximab. The safety of rituximab in combination with live viral vaccines has not been proven. Thus, such vaccinations are not recommended in patients during and after treatment with rituximab, when B-cell depletion is present. In the case of vaccination with inactivated viruses, response may be reduced during anti-CD20 treatment; it is therefore recommended to complete vaccination 4 weeks prior to starting treatment with rituximab.
No increase in the rate of infections in patients using rituximab compared to the control group has been reported; respiratory and urinary infections and pharyngitis are the most common.48 It has recently been shown that patients with pemphigus vulgaris treated with rituximab show a decrease in desmoglein antibodies (reactive antibodies), a phenomenon not observed with other immunosuppressors. In contrast, levels of antibodies against pathogens (such as varicella-zoster virus, Epstein-Barr virus, herpes simplex virus, pneumococcus, and the causative agent of tetanus) are increased. This surprising dual effect observed during rituximab treatment could be due to an increase in BAFF levels.29,49–51
- 5.
Less common adverse reactions. The less common adverse reactions to rituximab are as follows: psychiatric, ocular, neurologic (including progressive multifocal leukoencephalopathy, dizziness, and parasthesia), digestive, metabolic (increased LDH, increased blood glucose, and decreased calcium), hearing (tinnitus, ear pain), musculoskeletal, renal, cutaneous (Stevens-Johnson syndrome, toxic epidermal necrolysis, lichenoid dermatitis), and hematologic (pancytopenia) disorders.
In some patients, human antimurine antibodies (HAMA) and human antichimeric antibodies (HACA) develop during anti-CD20 treatment. In such cases, allergic or hypersensitivity reactions are more frequent if the patients are treated concomitantly with other monoclonal antibodies. The presence of such antibodies has been associated with the onset of serum sickness (skin manifestations, enlarged lymph nodes, fever, renal involvement, and peripheral neuropathy), though more so in autoimmune diseases than in hematologic malignancies.52 Fewer such side effects are expected in coming years with the introduction of fully humanized anti-CD20 antibodes.53 In view of the above, rituximab is a drug that has widespread potential applications in dermatology and a good 10-year safety profile.
Drug-Drug Interactions During Rituximab TreatmentTo date, no pharmacologic interactions have been reported when rituximab has been administered in combination with another drug, including immunosuppressants. The only factor to take into account is the possibility of the development of HAMA/HACA when other monoclonal antibodies are administered concomitantly, as discussed above. Use of rituximab with other immunosuppressive therapy should generally be avoided due to the greater risk of immunosuppression.
Special Patient GroupsIt is recommended that the reader consult the Summary of Product Characteristics available on the Internet: http://www.linfoma.roche.es/FichaTecnica Mabth.pdf.
IgGs can cross the placental barrier. While no formal studies in pregnant women treated with rituximab have been conducted, transient B-cell depletion and lymphopenia have been reported in some babies born to mothers exposed to rituximab during pregnancy. It is therefore recommended not to use rituximab in pregnant women; likewise, women are recommended to avoid becoming pregnant within 12 months of discontinuing the drug although studies in monkeys have not shown any indication of embryotoxicity in utero. An analysis of all isolated case reports in the literature indicates there is no increased risk of fetal toxicity.54 Until more reliable data are available, use of rituximab in pregnant women is initially be contraindicated although the benefits and risks should be assessed in each individual case. Rituximab is currently considered a category C product by the FDA.
It is not known whether rituximab is excreted into maternal milk, but maternal IgG is secreted in breast milk under normal conditions and the drug has been detected in the milk of rituximab-treated monkeys during lactation. Women receiving treatment with anti-CD20 antibodies are therefore advised not to breast feed for at least 12 months after discontinuation of treatment.
No large studies have examined the efficacy of rituximab in children. However, the literature points to the efficacy and safety of this drug in pediatric patients.55
Contraindications to Treatment With RituximabOn the basis of all information currently available, the following contraindications can be established for treatment with rituximab9:
- 1.
Pregnancy/lactation
- 2.
Age less than 18 years
- 3.
Sensitization to murine proteins
- 4.
Active hepatitis B or C virus infection
- 5.
HIV infection (CD4 cell count <250/mL)
- 6.
Severe heart failure
- 7.
Uncontrolled infection
As commented earlier, rituximab is currently only approved for use in patients with B-cell lymphomas, chronic lymphocytic leukemia, and rheumatoid arthritis. However, many published reports support the benefit of this drug, not only in other lymphoproliferative processes but also in an increasing number of autoimmune diseases. This beneficial effect is perhaps due to the mechanisms that underlie both types of disease. In many autoimmune processes, just as with B-cell lymphomas, B cells play an essential role, not only because of their ability to produce autoantibodies, but also because they can activate T cells and thereby amplify the immune response.
Table 1 lists all the conditions in which a beneficial response to rituximab has been reported; this section will only address dermatologic processes.
Conditions in Which a Beneficial Therapeutic Response Has Been Reported After Rituximab Administration.
| • Recurrent follicular non-Hodgkin lymphoma |
| • Chronic lymphocytic leukemia |
| • Rheumatoid arthritis |
| • Pemphigus vulgaris |
| • Foliaceus pemphigus |
| • Bullous pemphigoid |
| • Mucosal pemphigoid |
| • Acquired epidermolysis bullosa |
| • Paraneoplastic pemphigus |
| • Angioedema |
| • Anti-neutrophil-cytoplasmic-antibody–positive vasculitis |
| • Cryoglobulinemia |
| • Atopic dermatitis |
| • Chronic graft-versus-host disease |
| • Dermatomyositis |
| • Antiphospholipid syndrome |
| • Systemic lupus erythematosus |
| • Thrombotic thrombocytopenic purpura |
| • Idiopathic thrombocytopenic purpura |
| • Immunoglobulin-M-mediated neuropathies |
| • Cold agglutinin disease |
| • Hemophilia A |
| • Sjögren syndrome |
| • Multiple sclerosis |
| • Graves disease |
| • Autoimmune hemolytic anemia |
| • Waldenström disease |
Primary cutaneous B-cell lymphomas and benign cutaneous lymphoid hyperplasia represent the clearest dermatologic indications for the use of rituximab; indeed, they were the first cutaneous disorders for which this drug was indicated. Several studies support the use of the drug in these diseases. By way of example (and there are many), the study by Gellrich et al.56 seems particularly relevant. These authors reported a complete response in 70% of the patients treated in 8 weekly sessions with rituximab (375mg/m2) and established that this treatment is indicated primarily in patients with aggressive recurrent cutaneous B-cell lymphomas, particularly older patients and those with multiple skin lesions.
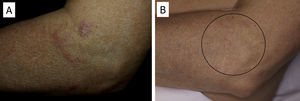
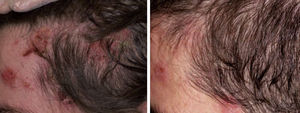
There have been isolated reports of cases of cutaneous B-cell lymphoid hyperplasia in which rituximab administration has been effective.40 We have also found this treatment useful in a patient with a pseudolymphoma in whom a clonal B-cell infiltrate was demonstrated (Fig. 5). In that case, the administration of rituximab in 4 weekly sessions (375mg/m2) led to complete resolution of the lesions, and the patient remained asymptomatic 2 years later.
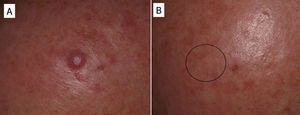
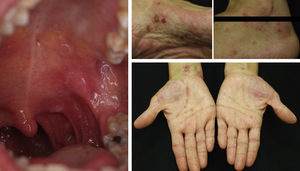
In some cases, resolution of primary cutaneous lymphomas has been observed after intralesional infiltration of rituximab. Fink-Puches et al.38 treated 8 patients with intralesional rituximab (10mg/mL, 1mL in each lesion), 3 times a week until an improvement in the lesions was obtained. The authors observed complete remission in 7 patients, with recurrences in 1 of these 27 months later. Recurrences occurred in untreated areas in 2 patients, 12 and 24 months later. The authors highlighted the disappearance of untreated lesions, probably due to absorption of the drug that subsequently acted in other areas. We have had the opportunity to treat a patient with marginal zone cutaneous B-cell lymphoma, which presented in the form of an isolated nodule in the scalp (Fig. 6). We performed 2 intralesional infiltrations (10mg/mL, 1mL in each session) 1 month apart. After 8 months, no clinical or histologic disease could be detected and clonality was negative.
Pemphigus VulgarisNumerous authors have described the effectiveness of rituximab in the treatment of pemphigus vulgaris. As yet, there are no established protocols for the use of the drug in this condition and there is no consensus regarding whether use of the drug is justified from the outset. Nevertheless, some authors have published guidelines based on their experience in which they suggest that anti-CD20 antibodies should be used when patients with pemphigus vulgaris do not respond to corticosteroids or immunosuppressants or when high doses of those agents are required to achieve a positive therapeutic effect.9,10,15,38 In most of the published cases, patients received concomitant treatment with immunosuppressants (azathioprine or mycophenolate) or immunomodulators (immunoglobulins).9 However, in our experience,46 which is in line with the findings of other groups, a single cycle of 4 weekly sessions (375mg/m2) in combination with corticosteroids can obtain complete remission in more than 85% of patients within 3 to 34 months.57 Normally, response occurs 6 to 10 months after completing the rituximab cycle, but may sometimes occur even later. This has been our experience (Fig. 7). The variability of the response may be determined by the patient's circulating CD20+ cell count.58
Finally, although the antidesmoglein 1 and 3 antibody titer usually correlates with pemphigus vulgaris activity, this is not always the case.46,58 A possible explanation for this inconsistency may be that rituximab's action is centered on the populations of B cells that produce antibodies against the most pathogenic epitopes, which are primarily CD20+ cells.
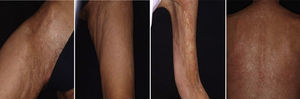
Acquired Epidermolysis BullosaThere are only a few reports of cases of acquired epidermolysis bullosa in which the administration of rituximab has been necessary to achieve disease control; Schmidt et al.59 reported an interesting case in which a combined regimen of rituximab, corticosteroids, immunosuppressants, and colchicine was required to control the disease. We have also used rituximab to control an inflammatory form of acquired epidermolysis bullosa in a patient with generalized lesions who experienced premenstrual exacerbations (Fig. 8). As these outbreaks were not controlled with a combined regimen of corticosteroids, mycophenolate, dapsone, and colchicine, we decided to add rituximab. After 6 months, the lesions disappeared and the anti-collagen VII antibody status was negative.
Bullous Pemphigoid and Mucosal PemphigoidIn many patients, bullous pemphigoid or mucosal pemphigoid can be controlled with conventional treatment. However, therapeutic alternatives are sometimes necessary because of a lack of response or side effects with conventional treatment. Lourari et al.60 reported their experience in 5 patients with bullous pemphigoid and 2 patients with mucosal pemphigoid. Complete remission was achieved by 3 patients with bullous pemphigoid and 1 with mucosal pemphigoid, although in 1 case the lesions recurred after 4 months.
Graft Versus Host DiseaseTogether with pemphigus vulgaris, graft-versus-host disease is perhaps one of the most promising indications for rituximab within dermatology. Recently, B cells have been implicated in the pathogenesis of this disease in which antibodies against platelet-derived growth factor are present. These antibodies can trigger an inflammatory cascade in the endothelium that eventually leads to cutaneous fibrosis. Kim et al.61 proposed the administration of rituximab (weekly infusions for 4 weeks followed by a monthly infusion for 4 months). Of the 37 patients treated according to this protocol, 8 achieved complete response and 24 achieved partial response, with a substantial decrease in the corticosteroid dose. The clinical improvement was much greater in the cutaneous, oral mucosal, and musculoskeletal manifestations. In our experience, in a woman with chronic graft-versus-host disease, treatment with rituximab led to a substantial improvement in both cutaneous and mucosal manifestations and musculoskeletal manifestations, almost halving the overall morbidity (Fig. 9).
DermatomyositisRecently, reports have been published of small series of patients who showed clinical benefit and improvement in laboratory parameters on treatment with rituximab.62 Levine published his experience in 6 patients affected by dermatomyositis. The rationale for administering rituximab was based on the role that B cells play in this disease. All patients experienced clinical improvement (functional improvement) and improved laboratory parameters approximately 3 months after starting treatment. In 4 cases, the lesions recurred after 52 weeks. No treatment-related side effects were reported. At this time, there are no specific guidelines for rituximab treatment in dermatomyositis (for example, a dose of 375mg/m2 weekly for 4 weeks or 1g administered on days 0 and 15). Larger series are needed to confirm the above findings. At present, a multicenter study of placebo versus rituximab is ongoing in patients with polymyositis/dermatomyositis and refractory myositis to address the open questions.
Atopic EczemaAnother condition for which there are literature reports of rituximab treatment is atopic eczema. Simon et al.37 administered 1000mg of rituximab in 2 sessions 15 days apart. Their study included 8 patients with severe atopic eczema. All skin lesions improved between 4 and 8 weeks after starting treatment, and a decrease from 30 to 9 was found in the eczema area severity index over a 6-month period. A decrease in spongiosis, acanthosis, and cutaneous hyperkeratosis was also observed. Circulating B cells disappeared completely in these patients, whereas their levels decreased by only 50% in samples from skin biopsies. Finally, the authors reported that although allergen-specific IgE levels remained unchanged, total IgE levels decreased. They concluded that rituximab could be a very important treatment for severe forms of atopic eczema that are refractory to other immunosuppressive agents.
Cryoglobulinemia-Associated VasculitisWink et al.63 reviewed all cases of cryoglobulinemia-associated vasculitis treated with rituximab. Complete remission was achieved in 60% and partial remission in 23%. The remaining patients showed no clinical response. In all cases, a significant reduction in cryoglobulins, rheumatoid factor, and IgM was observed. Complications were reported in patients with the highest cryoglobulin levels, in whom higher doses of rituximab were used (doses of 1000mg), and in patients with marked complement activation.
In patients with active hepatitis C virus infection and cryoglobulinemia, first-line treatment is pegylated interferon along with ribavirin. Administration of rituximab is recommended in cases of severe vasculitis, skin ulcers, neuropathy, and nephropathy.64
Acquired AngioedemaThere have been few reports of acquired angioedema treated with rituximab, in part because the condition itself is rare. Investigators have reported that patients with this condition can show a decrease in the frequency of outbreaks after 3 to 4 months of treatment, along with a decrease in the requirements for plasma protein C1 inhibitors, and a normalization of C4 levels.65
Antiphospholipid SyndromeIsolated cases of severe antiphospholipid syndrome treated with rituximab have been reported, with patients showing an improvement.66 However, it is too early to affirm that this could be one of the indications for rituximab, and we must await more extensive experience.67
Systemic Lupus ErythematosusThe efficacy of rituximab has been demonstrated in the treatment of skin manifestations of SLE. By way of example, according to data from the national French registry on the use of rituximab in SLE (136 patients), overall response was achieved by 71% of the patients; improvement was reported in joint manifestations in 72%, in skin manifestations in 70%, in renal manifestations in 74%, and in hematologic manifestations in 88%. Among the responders, 41% had a relapse, and the response rate among those initial responders was 91% after retreatment with rituximab. As in other diseases, the efficacy was not substantially different in patients who received rituximab monotherapy and those who received concomitant immunosuppressants (these patients had higher baseline disease activity).68
Perspectives and ConclusionsThe introduction of rituximab has opened up new perspectives in medical therapy, and specifically, within dermatology. Rituximab was first used for treating pemphigus vulgaris, though the agent has since been of great use in other skin conditions. To understand the potential scope of its application and whether rituximab may be effective in a given condition, it is important to understand how the immune response functions in these diseases. The action of rituximab has also helped us extend our knowledge of the pathogenesis of these conditions. As explained above, our current understanding would suggest that rituximab would not only be effective in conditions with direct autoantibody mediation, but also in those with a central role for B cells.
The outlook for drugs that act on molecular targets associated with B cells is promising. The incorporation of humanized anti-CD20 antibodies such as ofatumumab53 should help reduce the side effects associated with rituximab. In addition, studies are ongoing of antibodies (such as bortezomib)69 against long-lived plasma cells (negative for C20) which elude the effects of rituximab, and against agents that modulate B-cell activity, such as interleukin 6 (tocilizumab),70 BAFF (belimumab),71 and other B-cell receptors such as CD22 (epratuzumab).72 This pharmacologic arsenal will open the door to new therapeutic strategies in the coming years for conditions in which, at times, conventional treatment may fail or be associated with substantial side effects.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: España A, et al. Rituximab en dermatología. Actas Dermosifiliogr. 2013;104:380-92.