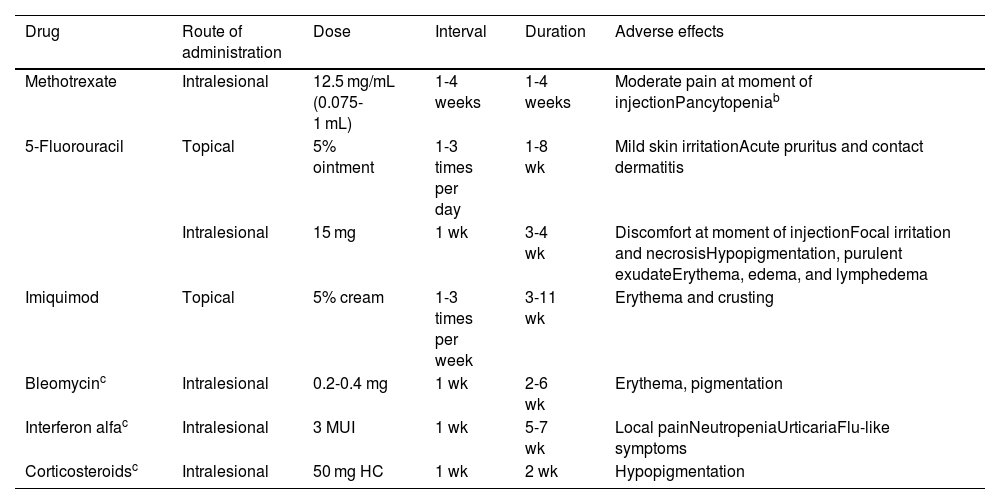
Keratoacanthoma (KA) is a skin tumor that, unlike classic cutaneous squamous cell carcinoma (SCC), grows rapidly and can subsequently partially or completely resolve. While some evidence supports its classification as a well differentiated variant of SCC, this remains a topic of debate. Clinical differentiation from SCC can be difficult, but it is useful to distinguish one from the other, as there are nonsurgical therapeutic options (Table 1) that may be useful for the treatment of KA, including intralesional infiltration of methotrexate (MTX) or 5-fluorouracil (5-FU), as well as topical application of imiquimod or 5-FU.1,2 Other, less frequently used intralesional substances, for which less supporting data are available, include bleomycin, corticosteroids, and interferon alfa.3
Nonsurgical Alternatives for the Management of Keratoacanthoma.a
| Drug | Route of administration | Dose | Interval | Duration | Adverse effects |
|---|---|---|---|---|---|
| Methotrexate | Intralesional | 12.5 mg/mL (0.075-1 mL) | 1-4 weeks | 1-4 weeks | Moderate pain at moment of injectionPancytopeniab |
| 5-Fluorouracil | Topical | 5% ointment | 1-3 times per day | 1-8 wk | Mild skin irritationAcute pruritus and contact dermatitis |
| Intralesional | 15 mg | 1 wk | 3-4 wk | Discomfort at moment of injectionFocal irritation and necrosisHypopigmentation, purulent exudateErythema, edema, and lymphedema | |
| Imiquimod | Topical | 5% cream | 1-3 times per week | 3-11 wk | Erythema and crusting |
| Bleomycinc | Intralesional | 0.2-0.4 mg | 1 wk | 2-6 wk | Erythema, pigmentation |
| Interferon alfac | Intralesional | 3 MUI | 1 wk | 5-7 wk | Local painNeutropeniaUrticariaFlu-like symptoms |
| Corticosteroidsc | Intralesional | 50 mg HC | 1 wk | 2 wk | Hypopigmentation |
Abbreviations: HC, hydrocortisone; MUI, million international units.
We always recommend histological confirmation before indicating nonsurgical treatment, as it can be difficult to clinically differentiate keratoacanthoma from cutaneous squamous cell carcinoma.
Despite the fact that no moderate or serious adverse effects have been recorded in the majority of patients, pancytopenia after treatment with intralesional methotrexate has been described in 2 patients with chronic kidney disease. It is therefore recommended to perform laboratory tests before and after drug infiltration.
Moss et al.4 recently published the largest study comparing intralesional MTX with conventional surgery in patients with a clinical or histological diagnosis of KA (n = 136; 157 tumors). In each case, the treatment decision was reached following consultation between the physician and the patient. In the 54 patients (n = 73) for whom intralesional MTX was indicated, a variable volume of 0.075-1 mL (depending on tumor size) was injected into the tumor base at a concentration of 12.5 mg/mL (25 mg/mL MTX diluted 50% with 1% lidocaine and 1:100 000 adrenaline). Doses were administered every 2-4 weeks. Complete resolution after 1 to 4 infiltrations (mean, 2.1) was observed in 88% (64 of 73) of KAs. Clinical cure was confirmed between 6 and 8 weeks after the last infiltration. The 9 patients who did not respond to intralesional MTX (8 KA lesions decreased in size and 1 remained unchanged) presented solitary KAs that were treated surgically, without complications. No moderate-to-severe adverse effects were observed in any of the patients treated with intralesional MTX. Mohs micrographic surgery was used for all of the 84 surgically-treated tumors, without recurrences or surgical complications. In an Italian series of 11 elderly patients with KA, mainly affecting the head and hands, weekly intralesional MTX infusions (median, 5.3; range, 4-8) resulted in complete resolution in all cases, without systemic adverse effects or recurrences after 6-9 months of follow-up.5
A 2019 systematic review of nonsurgically-treated KA (n = 184), not including the series by Moss et al.,4 found no significant differences in the rate of resolution between topical and intralesional treatments (92% and 100%, respectively), but reported a faster response for intralesional 5-FU versus intralesional MTX (3.7 vs 4.6 wk, which may not constitute a clinically significant difference) and for topical 5-FU versus topical imiquimod (3.8 vs 7.6 wk).1 Similar findings were reported in another recent review, with a 94% cure rate and no adverse effects of note.3
Spanish authors evaluated intralesional MTX as neoadjuvant therapy prior to KA surgery, and concluded that it is a well-tolerated measure that precludes the need for aggressive surgeries in facial or acral areas in elderly patients.2
Because spontaneous resolution of KA can take up to 1 year, or not occur at all, most authors recommend targeted treatment. Nonsurgical techniques (infiltrations and topical treatments) can be valid alternatives for KA located in complex areas (facial or acral) or in elderly patients.
Please cite this article as: Bosch-Amate X, Mancinelli C, Morgado-Carrasco D. FR - Tratamiento no quirúrgico de los queratoacantomas. Actas Dermosifiliogr. 2022;113:192–194.