Granuloma annulare (GA) is a dermatosis of unknown origin that has 3 characteristic histopathologic patterns: 1) necrobiotic granulomas in the mid-superficial dermis with 1 or more areas of necrobiosis with increased mucin deposition surrounded by histiocytes and lymphocytes, 2) interstitial (incomplete) form with increased lymphocytes and histiocytes among collagen bundles separated by mucin, and 3) tuberculoid or sarcoid granulomas.1 Clinical variants include localized, generalized, perforating, and subcutaneous GA, in addition to rarer variants such as palmar-plantar and patch forms.2 Localized GA is the most characteristic form and consists of ring-like pink or reddish papules and plaques, without an epidermal component, normally located on the extremities.
Of note among the histopathologic variants of GA are elastolytic granulomas, but GA may also occur in association with eccrine squamous syringometaplasia, mid-dermal elastolysis, and even vasculitis and neutrophils.2 Pseudolymphomatous GA is a more recently described variant.1
We report on a case of GA with pseudolymphomatous infiltrates as determined by histology.
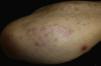
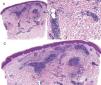
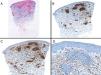
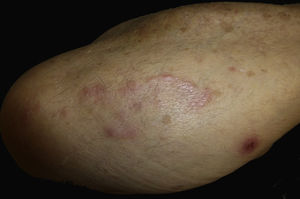
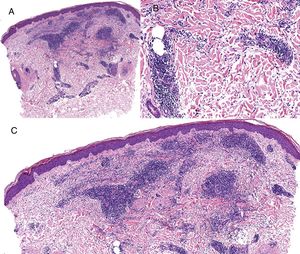
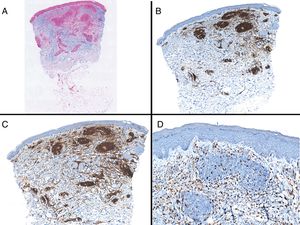
Case reportAn 82-year-old man with a history of hypertension, dilated myocardiopathy, and prostate cancer presented with a pruritic lesion of 1 year's duration on the dorsum of his right forearm. The physical examination showed erythematous papules that converged to form an arciform, nonindurated plaque measuring 5×2cm (Fig. 1). Biopsy showed an interstitial lymphocytic and histiocytic infiltrate accompanied by a prominent perivascular lymphocytic infiltrate without atypical cells. Alcian blue staining showed increased interstitial mucin deposition (Fig. 2A-C and Fig. 3A). Immunohistochemically, the perivascular infiltrate was formed by a mixture of CD4+ and CD8+ lymphocytes with numerous CD163+ and CD68+ cells among the collagen bundles (Fig. 3B-D). Groups of CD123-expressing dendritic plasmacytoid cells were not observed. Based on the clinical and histopathologic findings, a diagnosis of pseudolymphomatous GA was established. The lesion resolved completely after a month's application of topical propionate clobetasol 0.05% cream and there have been no recurrences to date. Pseudolymphomatous GA, which was described by Cota et al.1 in 2012, is histopathologically characterized by a dense lymphocytic infiltrate around the superficial and deep vessels, an absence of atypical lymphocytes, and concomitant interstitial or necrobiotic GA. The clinical findings in this first series of pseudolymphomatous GA suggested highly varied diagnoses (pseudolymphomas, mycosis, lichenoid dermatitis, sarcoidosis, papular dermatitis, plaque parapsoriasis, and figurate erythema), and an initial diagnosis of GA based on clinical findings was only made in 3 cases. Sixty percent of the patients had localized lesions similar to the one seen in our patient. The histopathologic differential diagnosis should include lymphoid hyperplasia, lupus tumidus (which can be ruled out by clinical findings and an interstitial pattern on biopsy), interstitial mycosis fungoides (histopathologic variant of mycosis fungoides characterized by lymphocytes scattered among collagen fibers and in which there are never more interstitial macrophages than lymphocytes), and interstitial granulomatous drug reaction, which we excluded based on the patient's history.
There have also been reports of granulomatous infiltrates, which can appear alongside specific atypical tumor cells, both in Hodgkin and non-Hodgkin lymphomas and in some solid tumors.3,4 In our patient, we were able to exclude this possibility with a high level of certainty given the rapid resolution of the lesion (typical in localized GA), the characteristic clinical findings, and the absence of concomitant disease. The association between GA and malignant tumors is probably fortuitous.5 There have also been reports of GA coexisting with lymphoid disorders, such as adult T-cell leukemia/lymphoma,3,6 acute myeloid leukemia, and primary cutaneous small to medium CD4+ T-cell lymphoma.4 Sarcoidosis and lymphoma could also be included in this second group, which we could consider to be nonspecific manifestations of lymphomas. A diagnosis of pseudolymphomatous GA should therefore be based on the integration of clinical and pathologic findings and be supported by immunohistochemical studies to rule out lymphoma and other tumors, particularly if atypical cells are observed. Serology and Borrelia polymerase chain reaction detection should also be performed to rule out borreliosis in endemic areas or in patients with compatible clinical manifestations. A diagnosis of pseudolymphomatous GA must be contemplated in cases of interstitial GA or GA with necrobiotic granulomas when a dense superficial and deep lymphoid infiltrate is observed. Pseudolymphomatous GA is rare and only a few cases have been reported in the literature. Familiarity with this entity is important to prevent overtreatment and unnecessary tests.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Llamas-Velasco M, Urquina-Renke A, Pérez-Plaza A, Fraga J. Pseudolymphomatous Granuloma Annulare: A Little-Known Variant. Actas Dermosifiliogr. 2019;110:162–164.