Pemphigus herpetiformis (PH) is a rare variant of pemphigus, with an incidence of 6% to 7.3% within the pemphigus population. Jablonkska et al. established the diagnostic criteria for PH in 1975, although similar cases had been described in 1955 under the name of dermatitis herpetiformis (DH). PH is characterized clinically by similarity with DH with an autoimmunity pattern that is similar to pemphigus.1
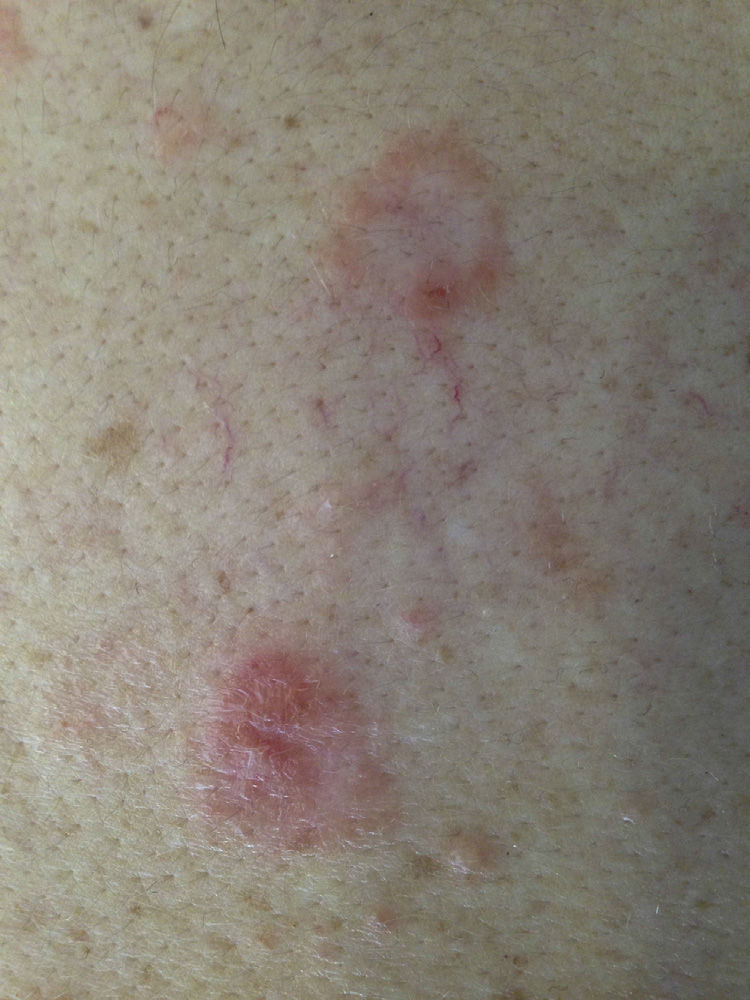
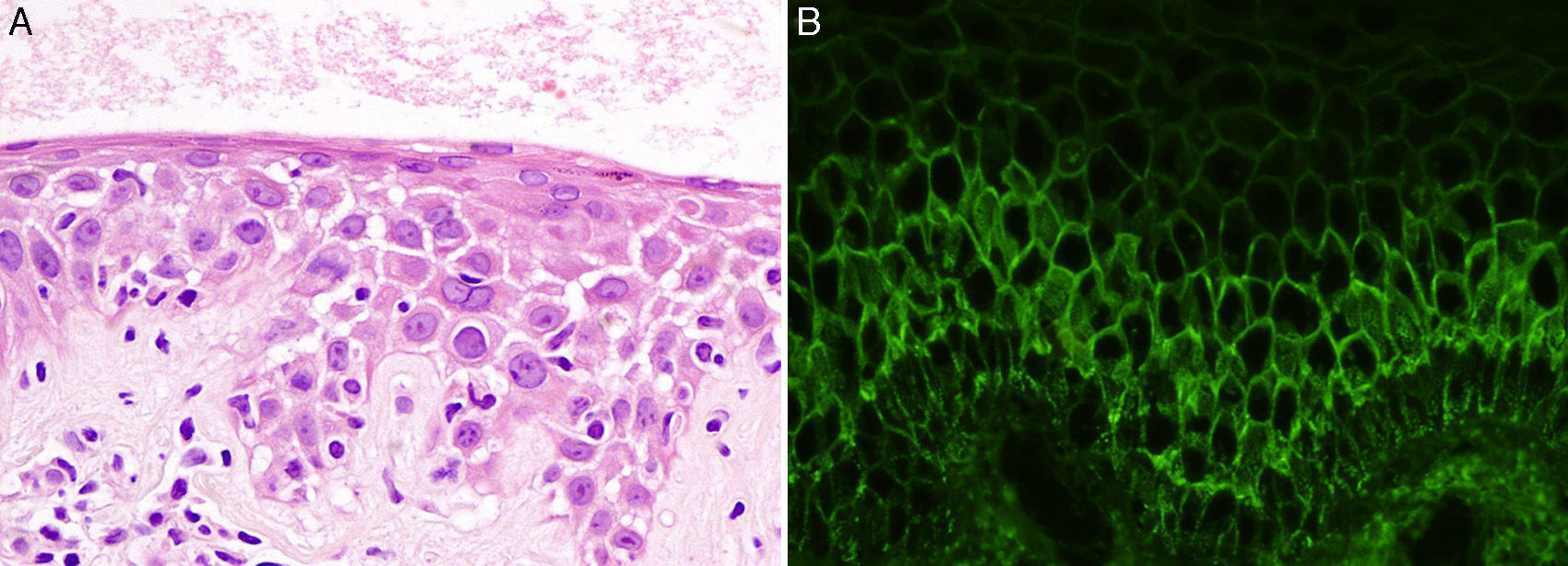
A 43-year-old woman with no personal or family history of interest was seen in dermatology outpatients for highly pruritic skin lesions localized mainly on her back. Physical examination revealed annular plaques of up to 3cm in diameter, formed of erythematous papulovesicular lesions of 1-2mm diameter, with a herpetiform distribution on the patient's back (Fig. 1). There was no mucosal involvement and the Nikolsky sign was negative. Histology of a lesion showed neutrophilic spongiosis. Direct immunofluorescence revealed intercellular deposits of immunoglobulin (Ig) G and was negative for C3 (Fig. 2). Additional tests, including complete blood count, routine biochemistry with liver and kidney profiles, serology for hepatitis B, hepatitis C, and human immunodeficiency virus, were normal or negative. The study of autoimmunity by enzyme-linked immunosorbent assay (ELISA) was positive for antidesmoglein 1 antibodies at a titer of 1:80 (normal value, < 1:10) and for antidesmoglein 3 antibodies at a titer of 1:30 (normal value, < 1:10).
Correlating the clinical manifestations, histology, and autoimmunity pattern, we were able to make a diagnosis of PH.
After the detection of normal levels of glucose-6-phosphate dehydrogenase, treatment was started with oral dapsone, 100mg/d, combined with prednisone, 60mg/d. The skin lesions and pruritus resolved within a few days. Tapering of the dose of prednisone was then initiated, with complete withdrawal 6 months after starting the treatment. After a year of follow-up, the patient remains asymptomatic and is only receiving treatment with dapsone, 100mg/d.
The clinical manifestations of PH are similar to those of DH. It is characterized by papulovesicular lesions on an erythematous base with a predominantly annular morphology. The lesions occur mainly on the trunk and proximal regions of the limbs and they are highly pruritic. In contrast to pemphigus vulgaris, mucosal involvement is very rare.1,2 An important finding on histology is the presence of spongiosis with subcorneal microabscesses without acantholysis. Neutrophils predominate in the inflammatory infiltrate in 20% of cases, eosinophils in another 20%, and a mixed infiltrate of neutrophils and eosinophils is seen in 60%. Neutrophilic spongiosis is an early finding in PH, but when acantholysis is present, it indicates a late phase.1,3 Direct immunofluorescence in PH reveals intercellular deposits of IgG and, less frequently, of C3. The antibody profile against desmosomal proteins detected by ELISA shows mainly antidesmoglein 1 and, less commonly, antidesmoglein 3.1 Interestingly, in the study by Ishii et al.4 with 20 samples from patients with PH, 16 were positive for antidesmoglein 1 and 4 for antidesmoglein 3, but none was positive for both antibodies. In the literature reviewed, our case stands out for being the first to be positive for antibodies to both antidesmoglein 1 and 3. The antibodies in pemphigus vulgaris and pemphigus foliaceous are pathogenic and comply with the desmoglein compensation theory, but this theory is not satisfied in PH.5,6 This is because the antibodies in PH recognize a less important segment of the protein and thus have a lower potential to induce acantholysis.4 As described in previous cases, the absence of mucosal involvement in our patient despite the presence of antidesmoglein 3 antibodies may also indicate that the antibodies in PH are less pathogenic. However, we cannot rule out that the absence of mucosal involvement was due to the low titers of antidesmoglein 3. Recently, new cases of PH with a different immunological profile have been published, with detection of antidesmocollin antibodies, with or without antidesmoglein antibodies.2,7
The differential diagnosis of PH includes DH, pemphigus foliaceous, IgA pemphigus, bullous pemphigoid, and linear IgA dermatosis.1,5 Associations have been reported between PH and malignant tumors and autoimmune diseases, but this association is rare and no firm relationship has been demonstrated. PH is a disease with a good prognosis and usually responds to the use of dapsone at a dose of 100-300mg/d. Dapsone is therefore considered first-line therapy in PH, sometimes combined with a systemic corticosteroid.1
In conclusion, we would like to stress that knowledge of PH is important as it has a different treatment and prognosis from pemphigus vulgaris. Our case is the first in the literature we consulted to show positivity for both antidesmoglein 1 and 3 antibodies. The absence of mucosal involvement despite the presence of antidesmoglein 3 antibodies can be explained by the lower pathogenicity of the antibodies in PH.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to thank Dr. Andrés Sanz Trelles for his help in the histological study and diagnosis of our case, and to the DERMACHAT Online Consensus Group for their collaboration in the diagnosis and treatment of our patient
Please cite this article as: Galvañ-Pérez del Pulgar JI, Tercedor-Sánchez J, Jiménez-Gallo D, Linares-Barrios M. Pénfigo herpetiforme con anticuerpos anti-desmogleína 1 y 3. Actas Dermosifiliogr. 2016;107:785–786.