Atopic dermatitis (AD) is a chronic inflammatory skin disease with symptoms such as pruritus that can be a major burden for patients. Patient-reported outcomes (PRO) complement clinician-reported outcomes in AD. This systematic review aims to identify and describe patient-reported outcome measures (PROM) used in observational studies of AD over the last decade in Spain. Eighteen PROM were identified to measure 13 different PRO that assess multiple aspects of the disease, including symptoms and disease severity, impact on daily activities and on work productivity/functioning, psychosocial impact, patient empowerment, and health-related quality of life (HRQoL). HRQoL, symptoms (particularly pruritus), and anxiety/depression were the most frequently assessed PRO, and the Dermatology Quality of Life Index, the Visual Analogue Pruritus Scale, and the Hospital Anxiety and Depression Scale were the most frequently used PROM, respectively. The growing number of observational studies on AD including PROM in Spain suggests that PRO are becoming increasingly important in the management of AD.
La dermatitis atópica (DA) es una enfermedad cutánea inflamatoria crónica con síntomas tales como el prurito, que pueden causar una carga significativa en la vida del paciente. Los resultados percibidos por los pacientes (PRO) complementan a los resultados clínicos evaluados en la DA por los médicos. Esta revisión sistemática tiene como objetivo identificar y describir las medidas de los resultados percibidos por los pacientes (PROM) utilizadas en estudios observacionales de DA durante la última década en España. Se identificaron 18 PROM para medir 13 PRO diferentes que evalúan múltiples aspectos de la enfermedad, incluyendo los síntomas y la gravedad de la enfermedad, la interferencia con las actividades diarias, el impacto psicosocial y laboral, el empoderamiento del paciente y la calidad de vida relacionada con la salud (CVRS). La CVRS, los síntomas (principalmente el prurito) y la ansiedad/depresión fueron los PRO más evaluados, siendo el Dermatology Life Quality Index, la Escala Visual Analógica del prurito y la Hospital Anxiety and Depression Scale, las PROM más frecuentemente empleadas, respectivamente. El número creciente de estudios observacionales sobre DA que incluyen PROM en España sugiere un aumento de la importancia de los PRO en el manejo de la DA.
Atopic dermatitis (AD) is a common chronic inflammatory skin disease, affecting 15.5% of children1 and 7.2% of adults in Spain.2 It is characterised by intense pruritus and recurrent eczematous lesions.3 AD presents symptoms that may lead to interference in daily activities, sleep disturbances, skin pain, impairment of psychosocial and work functioning, and impact on health-related quality of life (HRQoL),4,5 causing a significant burden on patient's life.6
Unlike other disorders, for most dermatological diseases no objective marker of the disease progression is available.7 Consequently, different clinician-reported outcome (ClinRO) measures have been developed to assess clinical signs, and their severity and extent in AD, e.g. Eczema Area and Severity Index (EASI), Investigator Global Assessment (IGA), validated IGA for AD (vIGA-AD™), SCORing Atopic Dermatitis (SCORAD). The latter also includes patient's perspective by evaluating the main symptoms of AD (i.e. pruritus and sleep disturbances). Assessing symptoms and their impact on daily activities, work productivity/functioning and HRQoL from patient's perspective is crucial to obtain a holistic view of the impact of the disease and determine its real burden.5,7 Therefore, patient-reported outcomes (PROs) are an essential complement to ClinRO in AD.5 For this reason, several patient-reported outcome measures (PROMs) have been developed during the last decades and most recently integrated in the development and evaluation of new treatments for AD.5
PROMs are also an integral part of clinical trial programmes in AD. In recent years, a global initiative has appeared to Harmonize Outcome Measures for Eczema (HOME)8 in clinical trials. This initiative has defined a core outcomes set and instruments including, among others: EASI, to measure the signs of the disease; the Patient-Oriented Eczema Measure (POEM), to collect patient-reported symptoms; Numerical Rating Scale (NRS), for measuring the peak pruritus over the past 24h; and Dermatology Life Quality Index (DLQI), to assess HRQoL.8 HOME is currently working on defining a list of suitable instruments for measuring health domains in clinical practice, where 5 domains have been agreed as the principal ones: PRO, disease/flare control, patient global assessment, clinician-reported signs, and eczema specific QoL. POEM, the Patient-oriented SCORAD index (PO-SCORAD), or both have been selected so far for measuring AD symptoms in clinical practice. Additionally, pruritus NRS has been provisionally recommended as an instrument for measuring pruritus intensity.6
Many different PROMs are available to assess PROs in AD in clinical practice, most of which focus on the symptoms and HRQoL impairment, but none comprehensively assesses all aspects of the disease. Due to this variety of instruments, several international reviews of PROMs for AD are available in the literature,9–11 but to date, none has reviewed the instruments used in Spain for AD. We conducted a systematic review to identify and describe specific PROMs used in AD studies conducted under real-world conditions during the last decade in Spain.
MethodsWe conducted a systematic review of the literature to identify studies that validated and/or used PROMs for AD in the context of clinical practice in Spain. Spanish (MEDES, IBECS) and international (PubMed/MEDLINE) databases were searched. This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations,12 and the recommendations set out in the Cochrane Handbook for Systematic Reviews of Interventions.13 Additionally, a broad search in the grey literature, including specific PRO research tools databases (BiblioPRO, PROQOLID), the Spanish (AEDV) and European (EADV) Dermatology congress websites, and Google/Google Scholar, was performed. Lastly, the reference lists of retrieved articles were reviewed to complement the systematic search.
The search strategy was focused on disease-related terms, PROs and PROMs, and country of interest (Table S1, Appendix additional material). The search was limited to original articles and congress communications published in English or Spanish, between April 1, 2010 and April 1, 2020. Publications that reported observational and/or epidemiological studies including Spanish patients with AD, describing the validation of PROMs or the use of tools to measure PROs in AD, were selected. Reviews and opinion articles, letters to the director and clinical trials were excluded from the review.
After removal of duplicates, two independent researchers selected the eligible publications applying the pre-defined inclusion and exclusion criteria. First, researchers screened by title and abstract, discarding publications outside the scope of the review. Full-text publications were subsequently reviewed. Discrepancies during the selection or evaluation process were resolved by consensus with the involvement of a third researcher. Two independent reviewers extracted data from selected publications.
Selected publications were categorised according to the study objectives in: “use of PROMs”, “use and validation of PROMs” and “validation of PROMs”.
Data from each study were extracted, including year of publication, study design, setting (national or multinational), population type (adults and/or children) and size, condition evaluated (AD or dermatological conditions including AD), PROs evaluated, and PROMs used.
For each PROM, extracted data included: outcome measure, type (generic/specific), target population, domains and/or aspects evaluated, number of items, period evaluated, score range, and the reference of the PROM validation or cross-cultural adaptation. Where information was not available from the retrieved publications, other available literature and PROMs developers’ official websites were reviewed.
Data were summarised descriptively with frequencies and percentages.
To assess the quality of the included observational studies we used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.14 The results of STROBE were calculated as the number of the 22 items adequately reported divided by the number of items, expressed as a percentage.
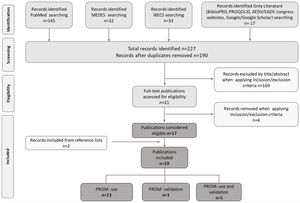
ResultsA total of 227 potentially relevant titles were initially recovered, 17 of which were considered eligible for inclusion. Two additional publications were identified during our search of key reference lists; hence 19 publications were finally included in the review. Of them, 13 reported the use of PROMs,2,15–26 five reported the use of PROMs and validation of at least one PROM in Spanish population,27–31 and one reported exclusively the validation of a PROM32 (Fig. 1).
Publication selection flowchart according to PRISMA. MEDES: MEdicina en ESpañol; IBECS: Índice Bibliográfico Español en Ciencias de la Salud; AEDV: Academia Española de Dermatología y Venereología; EADV: European Academy of Dermatology and Venereology; PROM: Patient-Reported Outcomes Measure.
The 19 publications included in the review described 17 different observational studies (Table 1) conducted in the last ten years. Most of these studies (n=11) were published in the 2018–2020 period. Sixty-five percent (n=11) of the studies had a cross-sectional design,2,17,19–21,24,25,28–31 24% (n=4) were retrospective,15,16,18,22,23,26,27 and 12% (n=2) were prospective.15,22,27 Regarding the geographical scope, 65% (n=11) were carried out only in Spain.15,16,18,19,21–23,26–29,31 Concerning the study population and the condition evaluated, 71% (n=12) included only adult patients,2,16–18,20,21,23–26,30,31 and 82% (n=14) only patients with AD.2,15–19,22–29,31,32 Lastly, regarding the quality of reporting, cross-sectional and prospective studies showed a high percentage of STROBE items adequately reported (77–100%), however case series studies reported percentages below 60%.16,18,23 STROBE was not evaluated in one study, for which only the abstract was available (Table 1).
Characteristics of the observational studies included in the review.
| Author (year) | Study design | Geography | Population type and size (n) | Condition | PRO evaluated | PROMs used | STROBE |
|---|---|---|---|---|---|---|---|
| Daudén et al. (2011)27, Sanchez Perez at al. (2013)15 | Prospective | Spain | Adults (172) and children (151) | AD | Pruritus | ISS | 19/2286% of items well-reported |
| HRQoL | DLQI, cDLQI | ||||||
| Torrelo et al. (2012)28 | Cross-sectional | Spain | Adults (159) and children (163) | AD | HRQoL | ADIS | 21/2295% of items well-reported |
| Torrelo et al. (2013)29 | Cross-sectional | Spain | Adults (141) and children (141) | AD | HRQoL | DLQI, cDLQI, iDLQI | 22/22100% of items well-reported |
| ADIS | |||||||
| Adherence | Morisky medication adherence scale | ||||||
| Satisfaction | VAS | ||||||
| Ortiz de Frutos et al. (2014)19 | Cross-sectional | Spain | Adults (125) and children (116) | AD | HRQoL | ADIS | 21/2295% of items well-reported |
| Disease control | Likert scale | ||||||
| Adherence | Likert scale | ||||||
| Dalgard et al. (2015)20 | Cross-sectional | Multinational (BE, DK, FR, DE, HU, IT, NL, NO, PL, RU, SP, TR, UK) | Adults (2) | Several dermatological conditions including AD | Anxiety/depression | HADS | 22/22100% of items well-reported |
| Marron et al. (2016)21 | Cross-sectional | Spain | Adults (11) | Several dermatological conditions including AD | Pruritus | ISS | 17/2277% of items well-reported |
| HRQoL | DLQI | ||||||
| Anxiety/depression | HADS | ||||||
| Family function | Family APGAR | ||||||
| Barbarot et al. (2018)2 | Cross-sectional | Multinational (FR, DE, IT, SP, UK, USA, CA, JP) | Adults (9924) | AD | Disease severity from the patient perspective | PO-SCORAD | 22/22100% of items well-reported |
| POEM | |||||||
| Patient Global Assessment | |||||||
| Ibáñez et al., (2018)22 | Prospective | Spain | Children (275) | AD | Pruritus | PO-SCORAD VAS | 22/22100% of items well-reported |
| Sleep disturbances | PO-SCORAD VAS | ||||||
| Benavente Villegas et al. (2018)23 | Retrospective case series | Spain | Adults (5) | AD | HRQoL | DLQI | 7/2232% of items well-reported |
| Zeidler at al. (2018)30 | Cross-sectional | Multinational (FR, DE, IT, PO, RU, SP, CH, TR) | Adults (27) | Several dermatological conditions including AD | HRQoL | ItchyQoL | 20/2291% of items well reported |
| Eckert et al. (2019)24 | Cross-sectional | Multinational (FR, DE, IT, SP, UK) | Adults (a) | AD | HRQoL | SF-36 (PCS and MCS) | 21/2295% of items well-reported |
| DLQI | |||||||
| Work productivity and activity impairment | WPAI | ||||||
| Ring et al. (2019)25, Arents et al. (2019)32 | Cross-sectional | Multinational (FR, SP, IT, UK, DE, NL, DK, SE, CZ) | Adults (180) | AD | Disease severity | POEM | 19/2286% of items well-reported |
| Self-assessment of severity | |||||||
| HRQoL | DLQI | ||||||
| Anxiety/depression | HADS | ||||||
| Emotional consequences | AESEC | ||||||
| Hernandez-Fernandez et al. (2019)26 | Retrospective case series | Spain | Adults (17) | AD | Pruritus | VAS | Not calculated (only abstract available) |
| Ruiz-Villaverde et al. (2019)16 | Retrospective case series | Spain | Adults (30) | AD | Pruritus | VAS | 13/2259% of items well-reported |
| HRQoL | DLQI | ||||||
| Pereyra-Rodriguez et al. (2019)31 | Cross-sectional | Spain | Adults (17) | AD | Patient Empowerment | DATEMP | 18/2282% of items well-reported |
| HRQoL | DLQI | ||||||
| de Bruin-Weller et al. (2020)17 | Cross-sectional | Multinational (FR, DE, SP, IT, UK, CA) | Adults (290) | AD | Pruritus | NRS | 19/2286% of items well-reported |
| PO-SCORAD VAS | |||||||
| Pain | NRS | ||||||
| Sleep disturbances | PO-SCORAD VAS | ||||||
| Question 2 of POEM | |||||||
| Question 4 of PSQI | |||||||
| Anxiety/depression | HADS | ||||||
| HRQoL | DLQI | ||||||
| Briceño Casado et al. (2020)18 | Retrospective case series | Spain | Adults (6) | AD | HRQoL | DLQI | 13/2259% of items well-reported |
3720 international patients (1860 with AD, 1860 without AD), 37% of the sample were Spanish patients. BE: Belgium; CA: Canada; CH: Switzerland; CZ: Czech Republic; DE: Germany; DK: Denmark; FR: France; HU: Hungary; IT: Italy; JP: Japan; NL: Netherlands; NO: Norway; PL: Poland; RU: Russia; SE: Sweden; SP: Spain; TR: Turkey; UK: United Kingdom; USA: United States; AD: Atopic dermatitis; ADIS: Atopic Dermatitis Impact Scale; AESEC: Atopic Eczema Score of Emotional Consequences; cDLQI: children Dermatology Life Quality Index; DATEMP: Dermatitis ATópica EMPoderamiento; DLQI: Dermatology Quality of Life Index; HADS: Hospital Anxiety and Depression Scale; HRQoL: health Related Quality of Life; iDLQI: infant Dermatology Life Quality Index; ISS: Itchy Severity Scale; MCS: Mental Component Scale; NRS: Numerical rating ScalePCS: Physical Component Scale; PO-SCORAD: Patient Oriented Scoring Atopic Dermatitis; POEM: Patient-Oriented Eczema Measure; PSQI: Pittsburgh Sleep Quality IndexSF-36: Short Form-36; VAS: Visual Analogue Scale; WPAI: Work Productivity and Activity Impairment.
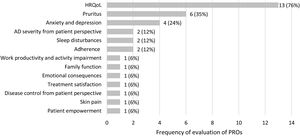
The 17 identified observational studies assessed 13 different PROs. The PROs most frequently evaluated were HRQoL, pruritus as a major symptom of AD, and anxiety and depression. Fig. 2 represents the frequency of evaluation of PROs in the studies included in the review.
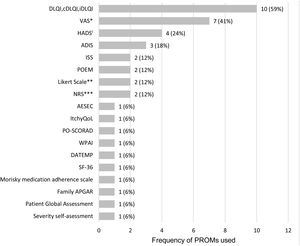
A total of 18 different PROMs were used, with DLQI and Visual Analogue Scales (VAS; three VAS and one PO-SCORAD VAS for pruritus, one VAS and one PO-SCORAD VAS for sleep disturbances) as the most frequently applied to assess HRQoL and the symptoms of the disease, respectively (Fig. 3, Table 2).
Frequency of PROMs used in the 17 observational studies. ADIS: Atopic Dermatitis Impact Scale; AESEC: Atopic Eczema Score of Emotional Consequences; cDLQI: children Dermatology Life Quality Index; DATEMP: Dermatitis ATópica EMPoderamiento; DLQI: Dermatology Quality of Life Index; HADS: Hospital Anxiety and Depression Scale; iDLQI: infant Dermatology Life Quality Index; ISS: Itchy Severity Scale; MCS: Mental Component Scale; NRS: Numerical rating ScalePCS: Physical Component Scale; PO-SCORAD: Patient Oriented Scoring Atopic Dermatitis; POEM: Patient-Oriented Eczema Measure; SF-36: Short Form-36; VAS: Visual Analogue Scale; WPAI: Work Productivity and Activity Impairment; *includes VAS for treatment satisfaction (1), VAS for pruritus (3), VAS for sleep disturbances (1); PO-SCORAD VAS for pruritus (1) and PO-SCORAD VAS for sleep disturbances (1); **includes a Likert scale for disease control and a Likert scale for treatment adherence; ***includes NRS for pruritus (1) and NRS for skin pain (1); iOne of the studies only evaluated the depression component of the questionnaire (HADS-D).
Characteristics of PROMs identified in observational studies involving AD patients in Spain.
| PRO category | PROM | Type | Target population | Domains and/or aspects evaluated | Items | Period evaluated (if available)/score range | PROM validation or cross-cultural adaptation |
|---|---|---|---|---|---|---|---|
| HRQoL | DLQI, cDLQI, iDLQI | Specific for dermatological conditions | Adults (DLQI), children (cDLQI) and infants (iDLQI) | Symptoms, function and emotions | 10 | Score range: 0–30 (0–1, no effect on patient's life; 2–5, small effect; 6–10, moderate effect; 11– 20, very large effect; and 21–30, extremely large effect) | DLQI: Developed in 1994 by Finlay et al.33 Transcultural adaptation into Spanish performed in 1998 by De Tiedra et al.51cDLQI: developed and validated in 1995 by Lewis-Jones et al.34.iDLQI: developed and validated in 2001 by Lewis-Jones et al.35. cDLQI and iDLQI have a Spanish translation available at http://www.cardiff.ac.uk/. |
| ADIS | Specific for AD | Adults and children | Patient's feelings and the impact of AD on social activities, leisure activities, and patients’ relationships28 | Adult version: 9Children version: 8 | Score range: 0 (minimum impact on HRQoL) – 10 (maximum impact on HRQoL) | Developed and validated in Spain in 2012 and 2013, respectively by Torrelo et al.28,29. | |
| ItchyQoL | Specific for pruritus | Adults | Symptoms, function and emotions | 22 | Score range: 0–110 (0–30, little impairment of HRQoL; 31–50, mild; 51–80, moderate; and 81–110, severe) | Developed and validated in 2008 by Desai et al36. Validated in Spain by Zeidler et al. in 201930. | |
| SF-36 | Generic | Adults | Physical Function; Physical Role; Body Pain; General Health; Vitality; Social Function; Emotional Role; Mental Health and 2 summary components (Physical and Mental) | 36 | Period evaluated: previous four weeksScore range: 0–100 scale, with higher scores indicating better health status | Developed in 1992 by Ware et al.38. Validated and translated into Spanish in 1995 by Alonso et al.37. | |
| Pruritus | ISS | Specific for pruritus | Adults and children | Frequency, intensity and extend of pruritus. Effect of pruritus on patient's mood, sexual life (adults) and sleep39 | Adult version: 7Children version: 6 | Score range: 0–21 (0=no presence of pruritus - 21=very severe pruritus) | Originally developed and validated in 2007 by Majeski et al.39. Validated in Spain in 2011 by Daudén et al.27. |
| VAS | Generic | Adults | Intensity of pruritus | 1 | Period evaluated: not specifiedScore range: 0–10 (0=no pruritus – 10=unbearable pruritus) | Validation information not available | |
| NRS | Generic | Adults | Intensity of pruritus | 1 | Period evaluated: the last 24hScore range: 0–10 (0=no pruritus – 10=worst pruritus imaginable) | Validation information not available | |
| PO-SCORAD VAS | Specific for AD | Adults and children | Intensity of pruritus | 1 | Period evaluated: last 3 daysScore range: 0–10 (0=no pruritus – 10=unbearable pruritus) | Validated in Europe in 2011 by Stalder et al.40.Spanish version available in https://www.poscorad.com/#/ | |
| Sleep disturbances | VAS | Generic | Adults | Presence of sleep disturbances | 1 | Score range: 0–10 (0=no trouble sleeping – 10=unable to sleep) | Validation information not available |
| PO-SCORAD VAS | Specific for AD | Adults and children | Presence of sleep disturbances | 1 | Period evaluated: last three daysScore range: 0–10 (0=no trouble sleeping – 10=unable to sleep) | Validated in Europe in 2011 by Stalder et al.40.Spanish version available in https://www.poscorad.com/#/ | |
| Question 4 – PSQI modifieda | Generic | Adults | PSQI includes component scores (10 items) for: Subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbances; use of sleeping medication; daytime dysfunction52De Bruin Weller et al.18 only used a modification of question 4 of the PSQI questionnaire (sleep duration): “during the past week, how many hours of actual sleep did you get at night?” | 1 | Period evaluated: last weekAverage hours of sleep during the last week18 | Originally developed and validated in 1989 by Buysse et al.52. Validated in Spain in 1997 by Royuela-Rico et al.53. | |
| Skin pain | NRS | Generic | Adults | Level of skin pain | 1 | Period evaluated: the last 24hScore range: 0–10 (0=no pain - 10=worst imaginable pain) | Validation information not available |
| Disease control from the patient perspective | Questionnaire based on Likert Scale | Generic19 | Adults and children | Degree of disease control | 1 | Score range: 1–6 (1=excellent disease control – 6=very poor control of the disease) | Developed in 2014 by Ortiz de Frutos et al.19. Validation information not available |
| AD severity from the patient perspective | PO-SCORAD | Specific for AD | Adults and children | Surface affected, severity of AD signs and intensity of symptoms | 9 | Period evaluated: last 3 days40Score range: 0–103: Surface affected and severity of signs (7 items) range 0–83; intensity of symptoms (2 items) range 0–20(0–24, mild AD; 25–49, moderate AD; ≥50, severe AD) | Validated in Europe in 2011 by Stalder et al.40.Spanish version available in https://www.poscorad.com/#/ |
| POEM | Specific for AD | Adults | Frequency of symptoms41 | 7 | Period evaluated: last 7 daysScore range: 0–28 (≤7, mild; 8–16, moderate; >16, severe) | Developed and validated in 2004 by Charman et al.41.Spanish version available (linguistic validation certificate available in www.nottingham.ac.uk) | |
| Patient Global Assessment | Generic | Adults | Severity of disease | 1 | Score range: mild, moderate or severe | Validation information not available | |
| Severity self-assessment | Generic | Adults | Severity of disease | 1 | Score range: clear, almost clear, mild, moderate or severe | Validation information not available | |
| Treatment satisfaction | VAS | Generic | Adults | Overall satisfaction. Degree of satisfaction with the prevention of flares, lengthening of flare-free intervals, frequency of treatment application, information received about maintenance therapy, and information received about treating flares during maintenance therapy | 1 | Score range: 0–10 (0=minimum satisfaction- 10=maximum satisfaction) | Validation information not available |
| Adherence | Morisky medication adherence scale | Generic | Adults | Treatment adherence | 4 | Score range: Patients were considered to have adhered to treatment if they answered “no” to questions 1, 3, and 4 and “yes” to question 2. | Developed and validated in 1986 by Morisky et al.43. Validated in Spain in 1992 by Val Jiménez et al.44. |
| Questionnaire based on Likert Scale19 | Generic19 | Adults and children | Patient adherence with the recommendations related to treatment, skin hydration, special soap use, avoidance of high temperatures, and food recommendations | 5 | Score range: 1–6 (1=always apply or comply with the indication - 6=rarely or never apply) | Developed in 2014 by Ortiz de Frutos et al.19. Validation information not available. | |
| Anxiety and depression | HADS | Generic | Adults | Anxiety and depression | 7 for anxiety+7 for depression | Period evaluated: last 7 daysScore range: 0–21 (0–7, normal; 8–10, presence of anxiety or depression symptoms; and 11–21, clinical anxiety or depression). | Developed in 1983 by Zigmond et al.46. Spanish validation conducted in 2003 by Herrero et al.45 |
| Emotional consequences | AESEC | Specific for AD | Adults | Effect of AD on the patient emotional status | 28 | Period evaluated: not time-specificScore range: 0–84 (0–27, ‘no/small effect’; 28–40, ‘moderate effect’; 40–52, ‘large effect’; and 53–84, a ‘very large effect’) | AESEC was developed and validated in two multi-country studies included in the review25,32. |
| Family function | Family APGAR | Generic | Adults | Adaptation, Partnership, Growth, Affection, and Resolve | 5 | Score range: Patients have three choices of response: “Almost always”, “Some of the time”, or “Hardly ever”. The patient checks one of three options which are scored as follows: ‘Almost always’ (2 points), ‘Some of the time’ (1 point), or ‘Hardly ever’ (0 points). The scores for each of the five questions are then totalled. A score of 7–10 suggests a highly functional family; 4–6 suggests a moderately dysfunctional family; 0–3 suggests a severely dysfunctional family | Developed in 1978 by G Smilkstein48. Validated in Spain in 1996 by Bellon-Saameño et al.47. |
| Work productivity and activity impairment | WPAI | Generic | Adults | Absenteeism, presenteeism, overall work impairment, and activity impairment | 6 | Period evaluated: last 7 daysScore range: WPAI outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity, i.e., worse outcomes. | Developed and validated in 1993 by Reilly et al.50 Linguistic validation into Spanish carried out in 2006 by Gawlicki et al.49. |
| Patient empowerment | DATEMP | Specific for AD | Adults | 4 dimensions: active self-care (skills), basic knowledge about the disease (knowledge), personal-social concerns (coping skills), and change intention (change). | 17 | Period evaluated: the questionnaire evaluates the patient empowerment in the present time.Score range: 17–85 (17, low level of empowerment; 85, high level of empowerment) | Pereyra-Rodriguez et al. developed and validated this questionnaire in Spanish population31. |
ADIS: Atopic Dermatitis Impact Scale; AESEC: Atopic Eczema Score of Emotional Consequences; cDLQI: children Dermatology Life Quality Index; DATEMP: Dermatitis ATópica EMPoderamiento; DLQI: Dermatology Quality of Life Index; HADS: Hospital Anxiety and Depression Scale; iDLQI: infant Dermatology Life Quality Index; ISS: Itchy Severity Scale; MCS: Mental Component Scale; NRS: Numerical rating ScalePCS: Physical Component Scale; PO-SCORAD: Patient Oriented Scoring Atopic Dermatitis; POEM: Patient-Oriented Eczema Measure; PSQI: Pittsburgh Sleep Quality IndexSF-36: Short Form-36; VAS: Visual Analogue Scale; WPAI: Work Productivity and Activity Impairment.
Only a modification of question 4 of PSQI questionnaire was used in de Bruin Weller et al.18 publication, and therefore, PSQI has not been counted among the identified PROMs. Details on the domains and/or aspects evaluated, items, period evaluated, score range and PROM validation or cross-cultural adaptation have been obtained from the literature.
To evaluate HRQoL, four different PROMs were used in thirteen observational studies, some of them being administered simultaneously in the same study (n=2). Three PROMs were specific HRQoL questionnaires: DLQI15–18,21,23,25,27,31 (or its child (cDLQI) and infant (iDLQI) versions), Atopic Dermatitis Impact Scale (ADIS)19,28,29 and ItchyQoL questionnaire.30 The other PROM identified was a generic HRQoL questionnaire: Short Form-36 (SF-36).24 DLQI, cDLQI and iDLQI are specific questionnaires for dermatological conditions composed of 10 items, which evaluate symptoms, feelings, and problems related to daily activities, leisure, work and school, personal relationships, and treatment.33–35 ADIS is a specific questionnaire for AD that can be administered to adults and children, composed of 9 and 8 items, respectively, which assess patient's feelings and the impact of AD on social activities, leisure activities, and patients’ relationships.28,29 ItchyQoL is a specific questionnaire for pruritus used in adults, composed of 22 items that evaluate symptoms, function and emotions.30,36 Finally, SF-36 is a generic questionnaire for adults, composed of 36 items evaluating physical function, physical role, body pain, general health, vitality, social function, emotional role and mental health. All these PROMs measure the impact of the disease on patients’ HRQoL.37,38
Symptoms and disease controlThree symptoms were evaluated in the studies identified, with pruritus being the most frequently assessed (six studies), followed by sleep disturbances (two studies) and skin pain (one study).
Pruritus was assessed in the studies using a single PROM (n=5) or two PROMs (n=1). Four different PROMs were employed: the Itchy Severity Scale (ISS),15,21,27 a VAS,16,22,26 a Numerical Rating Scale (NRS)17 and the PO-SCORAD VAS.17 ISS is a specific tool for pruritus in skin diseases that quantifies the frequency, intensity and extent of pruritus, and its effect on patients’ mood, sexual life and sleep, in adults and children through a 7- and 6-items questionnaire, respectively.27,39 The NRS and VAS are generic unidimensional scales administered to adults to evaluate the pruritus intensity, while the PO-SCORAD VAS has been developed and validated also for use in children.34 To assess the presence of sleep disturbances one study used a generic VAS,22 while another one used three different measures17: PO-SCORAD VAS, question 2 of POEM (“Over the last week, on how many nights has your sleep been disturbed because of your eczema?”), and a modification of question 4 of the Pittsburgh Sleep Quality Index (PSQI) (“During the past week, how many hours of actual sleep did you get at night?”). Finally, the level of skin pain was assessed in adults using a NRS.17
The degree of disease control from the patient perspective was assessed in one study using a questionnaire based on a 6-point Likert scale.19
AD severity from the patient perspectiveAD severity from patient perspective was evaluated in the studies with a single PROM (n=1) or three PROMs (n=1). The four PROMs identified were: PO-SCORAD,2 POEM,2 Patient Global Assessment (where the patient assessed the disease as mild, moderate or severe),2 and a self-assessment of the severity (clear, almost clear, mild, moderate or severe).25 The PO-SCORAD is a disease-specific tool that can be used in adults and children and comprises 7 items measuring the severity of AD signs (surface area affected by eczema in the last 3 days, dryness of the skin without eczema, evaluation of the severity of the eczema over the last 3 days), and two Visual Analogue Scales (PO-SCORAD VAS) to assess the intensity of symptoms (pruritus and sleep disturbances).40 POEM is a specific questionnaire designed for use in adult patients with AD, composed of 7 items that assess AD severity by evaluating the frequency of symptoms (dryness, pruritus, flaking, cracking, sleep loss, bleeding, weeping) per week.41 Although not identified in our review, a children's version is also available.42
Treatment satisfactionPatient satisfaction with maintenance therapy for AD was determined in one study using multiple VAS.29 Different aspects of satisfaction were evaluated, including overall satisfaction, satisfaction with the prevention of flares, satisfaction with lengthening of flare-free intervals, satisfaction with frequency of treatment application, satisfaction with information received about maintenance therapy, and satisfaction with information received about treating flares during maintenance therapy.
AdherenceTwo PROMs were used to assess adherence in two studies: the Morisky medication adherence scale,29 a validated generic questionnaire,43,44 was used to measure treatment adherence, while a questionnaire based on a 6-point Likert scale19 was employed to evaluate adherence to medical recommendations.
Psychosocial impactThe psychosocial impact of AD was evaluated in four studies using a single PROM (n=2) or two PROMs (n=2). The presence of anxiety and depression associated to AD was assessed through the Hospital Anxiety and Depression Scale (HADS).17,20,21,25 HADS is a generic questionnaire for adults composed of two subscales, one of them measuring the presence of anxiety (7 items) and the other depression (7 items).45,46 The Atopic Eczema Score of Emotional Consequences (AESEC), a specific questionnaire for adults with AD composed of 28 items, was used to determine the effect of AD on the patient emotional status.25 The psychometric properties of AESEC were reported in detail by Arents et al.32 The Family APGAR, a generic questionnaire for adults composed of 5 items,47,48 allowed to assess how family function (adaptation, partnership, growth, affection, and resolve) was affected by AD.21
Work productivity and activity impairmentThe work productivity (including absenteeism, presenteeism, overall work impairment, and activity impairment) was evaluated in one study using Work Productivity and Activity Impairment (WPAI),24 a generic questionnaire for adults composed of 6 items.49,50
Patient empowermentThe level of patient empowerment was assessed in one study using the Dermatitis ATópica EMPoderamiento (DATEMP), a specific questionnaire for adults with AD, originally developed and validated in Spanish, composed of 17 items, which evaluates aspects such as active self-care, basic knowledge about the disease, personal-social concerns, and intention to change.31
DiscussionThis review identified and described PROMs used in observational studies in AD in Spain during the last 10 years to assess key aspects of the disease from the patients’ perspective. The identified PROMs assessed the occurrence of symptoms (e.g., pruritus) and disease severity, interference with daily activities, impact on psychosocial and work functioning, patient empowerment and HRQoL. This review provides dermatologists an overview of tools available to measure PROs in observational studies with applicability in clinical practice in Spain. The growing interest in measuring PRO outcomes to obtain the patient perspective is reflected in the results of the review, with more than half of the observational studies identified published in the last three years.
In total, eighteen different PROMs were identified: 55% were generic (applicable to a wide range of conditions), 6% specific for dermatological conditions (applicable to different skin disorders), 11% specific for pruritus (one of the main symptoms of AD), and one third specific for AD. Both specific measures for dermatology and AD provide a complementary view of affected areas/domains that are not assessed with generic instruments.
The PROMs identified assessed thirteen distinct patient outcomes, where HRQoL, symptoms (mainly pruritus) and anxiety/depression were the most frequently evaluated. These findings are in line with a previous systematic review identifying PROMs used in clinical trials to assess new treatments in AD, where tools to evaluate HRQoL and pruritus, were the most frequently employed.5
Our review shows that DLQI and its children and infant versions are also the most frequently used in observational studies in Spain. Despite not being an AD specific tool, DLQI has been widely used as a HRQoL measure in dermatology clinical trials, validated in AD patients, and included in HOME recommendations from 2017.9 Even though previous studies have shown a significant correlation between HRQoL measures such as DLQI and AD severity, there is scarce information about the routine use of HRQoL measures in the management of patients with AD.54 This review underlines the widespread use of DLQI in observational studies in dermatology in Spain. Moreover, its inclusion as an outcome suggests that it is feasible to integrate information about the impact of the disease and its symptoms on HRQoL under clinical practice conditions. Measuring tools such as DLQI may help to better assess how well the disease is being managed/controlled and the effectiveness of treatments beyond objective clinical assessment. Nevertheless, DLQI subscales have previously shown a considerable floor effect (which makes it difficult to detect worsening of perceived HRQoL),55 and last HOME systematic review of HRQoL measures indicated insufficient structural validity for DLQI to be recommended for use.11Some authors recommend adding a generic to a dermatology-specific PROM, to measure individuals’ health status in generic terms, and in situations where the skin disease has a substantial generic HRQoL impact beyond the disease-specific impact.57,58 Our review identified SF-36 as a possible available generic HRQoL tool for Spanish population.
According to the last HOME consensus,6 patient-reported symptoms is the highest-prioritized domain to measure in clinical practice in patients with atopic eczema. In our review, symptoms were measured as a stand-alone outcome (using VAS or NRS) or together with other aspects of the disease as part of a comprehensive PROM, such as PO-SCORAD, POEM or HRQoL questionnaires. Symptoms, as a stand-alone outcome, were evaluated in a third of the studies reviewed.15–17,21,22,26,27 Pruritus was assessed in all of them, with pruritus VAS as the most used PROM (67%), unlike the recommendations of HOME, that provisionally recommend a NRS as preferred tool to measure pruritus intensity. Sleep disturbances were evaluated in one third of the studies assessing patient-reported symptoms as a stand-alone outcome,17,22 and skin pain in 17%.17 Although they are the major symptoms in AD and a relevant domain for clinical trials, they are not yet seen as a standard measure for clinical practice. In a previous systematic review evaluating symptom reporting in clinical trials in AD, it was found that 78% of them used PROMs to assess symptoms, of which only 37% measured symptoms as stand-alone outcomes. Among them, pruritus was the most assessed symptom (98%), with VAS being the most used PROM (76%),56 followed by sleep disturbance (61%), which is largely considered a surrogate of pruritus.
Since in patients with moderate and severe AD the symptomatology can impact on patient's daily living activities and emotional wellbeing,15 measuring symptoms, especially pruritus, becomes a key point in the evaluation of the disease. Our review has identified both unidimensional (VAS or NRS) and multidimensional tools (PO-SCORAD or POEM) to evaluate pruritus. Although unidimensional scales and PO-SCORAD assess the intensity of itching, and POEM collects its frequency, multidimensional tools provide a more holistic view of the disease, and they have been previously correlated with the severity of AD and different dimensions of HRQoL.57 However, the use of these instruments in clinical practice is scarce and limited to clinical research, and according to HOME initiative, unidimensional scales are preferred as a simple and rapid method for pruritus intensity assessment in clinical practice.59–62
Our results show that the AD's psychosocial impact, including anxiety and depression, emotional consequences and family function are PROs evaluated in recent observational studies identified. Among them, anxiety and depression have been the most assessed PROs and HADS questionnaire the main PROM used. This is in alignment with the high burden of psychiatric-related disorders reported in the literature among patients with AD in Spain e.g. de Bruin-Weller et al. report the presence of emotional or mental comorbidity in 17–31% of patients with mild to severe AD, respectively, which increases with disease severity.17 Higher prevalence of anxiety and depression in patients with AD compared to patients without AD has been reported, associating worse HADS scores with worse disease severity.63 Additionally, Spanish patients experiencing chronic pruritus present statistically significant differences for the anxiety subscale of HADS compared to those who do not experience chronic pruritus.21 Because of the association between AD and psychiatric disorders, HADS could be a useful tool in clinical practice to assess the mental health of these patients.
Recently, an international study in adult patients with AD has evidenced the high impact on patient productivity and activity, with a greater negative impact on patients with moderate and severe disease.64 Reducing the severity of the AD can be expected to decrease the burden on the patient and therefore the impact on productivity and activity, which could be an aspect to be taken into account to assess in clinical practice. However, current review has identified only one study assessing the impact of AD on work productivity and activity through the WPAI questionnaire.
Finally, in our review, the Morisky medication adherence scale was the PROM chosen to evaluate patient adherence to treatment,29 while a Likert Scale was used to measure adherence to medical recommendations.19 To assess patient's satisfaction a VAS was employed.29 These results highlight the possible underestimation of the impact of adherence and patient satisfaction with treatment in AD patients, as only two publications evaluate them, relevant to assess the effectiveness of the prescribed treatment.
Despite the increasing number of studies evaluating PROMs in our review, the systematic use of tools to capture patient perception for a holistic management of AD in Spain is still scarce. The limited use of PROMs in clinical practice could be due to the reduced time in consultations, the lack of collaboration or inability of patients to complete PROMs, the lack of habit in incorporating the patient in decision making, and the current healthcare model where the patient's empowerment is low. Nevertheless, our results show that the use of PROMs in clinical practice could be increasing since the patient's perspective is becoming more relevant to the clinician in disease management. There is still scope for increased measurement of patient-reported symptoms and its consequences on different aspects of patient's life.
This review's main strength is the systematic approach used and the focus on PROs assessed in observational studies, which allowed to provide an overview of identified PROMs that may be applicable for use in routine clinical practice conditions. This review is not exempt of limitations. Although STROBE checklist was used to determine the quality of the included observational studies, the review did not analyse the possible biases of the included observational studies. In addition, the review included international studies involving Spanish patients with AD regardless of their sample size. Therefore, their results may not be an accurate representation of our setting. However, the review's objective was not to analyse the results obtained in those studies but to identify possible PROMs used in real-world studies that may have applicability in clinical practice. Lastly, our review was not designed to be a comprehensive review of all available PROMs but rather to identify PROMs used in real-world studies as these may provide a better reflection of routine clinical practice in Spain. As such, PROMs used in clinical trials and those not reported in observational studies including Spanish patients over the last decade were excluded.
ConclusionsThe number of real-world studies that include PROMs conducted in Spanish AD patients has increased over time, suggesting a growing importance of PROs in assessing and managing AD from a patient-centred point of view. Several validated PROMs are available for use in Spain, covering the main aspects of the disease, including symptoms (e.g. pruritus) and disease severity, interference with daily activities, impact on psychosocial and work functioning, patient empowerment and HRQoL. However, there is still scope for increasing the measurement of patient-reported symptoms to understand their consequences and impact on patient's life in terms of disease progression and treatment effectiveness.
Conflicts of interestLilly Spain has financed the publication of this article.