Plaque psoriasis, also known as psoriasis vulgaris, is a chronic inflammatory disease characterized by erythematous scaly plaques of varying severity that form on the surface of the skin.1 The estimated prevalence in Spain is 2.3%; 55.6% of patients have mild plaque psoriasis, 37.6% moderate, and 6.6% severe.2,3
Topical treatment is one of the mainstays of psoriasis management.4 It is generally recommended for mild to moderate psoriasis and can be used as an adjuvant to systemic therapy and/or phototherapy in patients with moderate to severe disease.5 Patient satisfaction with topical treatments, however, is lower than that generally reported for other treatments, and this affects treatment adherence. Alternative topical treatments that meet patients’ needs and ensure proper adherence are needed.6
The reformulation of known active ingredients into a new vehicle is a promising strategy for improving treatment effectiveness and convenience.7 One such example is the fixed combination of the vitamin D analog calcipotriol (Cal) and betamethasone dipropionate (BD) in an aerosol foam.8 Clinical trials have shown that Cal/BD aerosol foam has superior efficacy to Cal and BD used in isolation9 and to Cal/BD in ointment10,11 and gel12 formulations, while offering a similarly favorable safety profile.13,14
Cal/BD in aerosol foam is more effective than Cal/BD in other formulations because the active ingredients are dissolved in volatile propellants that evaporate when the product is applied to the skin. This creates a foam layer containing Cal/BD at a higher concentration than their maximum solubility point (supersaturation), enabling enhanced penetration of the drug through the epidermis.15,16
One way of improving adherence to topical treatments in psoriasis is to improve patient satisfaction. We conducted an observational study, the first of its kind, to assess satisfaction among both patients and physicians with Cal/BD aerosol foam for the treatment of plaque psoriasis on the body in routine practice in Spain.
Material and MethodsStudy DesignWe performed a multicenter, cross-sectional, observational study in 88 Spanish hospitals and clinics between October 2017 and March 2018. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Clinical Research Ethics Committee at Hospital Arnau de Vilanova in Valencia, Spain. All patients gave their written informed consent.
Data on treatment satisfaction were collected in a single visit in which patients completed a questionnaire and physicians rated their satisfaction on a Likert scale. Clinical and sociodemographic data were collected retrospectively from the patients’ medical records.
Study PopulationPatients aged over 18 years with plaque psoriasis covering a maximum body surface area of 30% were included. Additional inclusion criteria were treatment with Cal/BD aerosol foam in the 4 weeks leading up to the study and treatment with a topical product other than the foam in the previous 6 months.
Patients treated with systemic or biologic therapy at any time between 4 and 16 weeks before treatment with Cal/BD aerosol foam were excluded, as were patients with comprehension difficulties.
Study VariablesPatient satisfaction was assessed using the abbreviated version of the Treatment Satisfaction Questionnaire for Medication (TSQM-9), which compares satisfaction with current and past topical treatments. Physician satisfaction was assessed using a 5-point Likert scale.
The abbreviated version of the TSQM questionnaire contains 9 items divided into 3 domains: effectiveness (3 items scored on a scale of 1-7 [extremely satisfied to extremely dissatisfied]), convenience (3 items scored on a scale of 1-7 [extremely easy to extremely difficulty]), and global satisfaction with treatment (2 items related to confidence in treatment scored on a scale of 1-5 [extremely confident/certain to not at all confident/certain] and 1 item on overall satisfaction scored on a scale of 1-7 [extremely satisfied to extremely dissatisfied]).17 The patients completed the questionnaire during the study visit and results are reported as the percentage of patients for each category.
Physicians rated their satisfaction with Cal/BD aerosol foam on a 5-point Likert scale ranging from very satisfied to not at all satisfied for 5 treatment-related domains: improvements in symptoms, emotional well-being, and adherence, satisfaction with foam vehicle, and global satisfaction. Again, the results are reported as the proportion of physicians for each category.
Statistical AnalysisA descriptive analysis of the study variables was performed. Continuous variables are reported as mean [SD] and median, while categorical variables are reported as number and percentage of cases and distribution of frequencies. Statistical analyses were performed using the SPSS statistical package, version 9.2 for Windows.
ResultsStudy PopulationA total of 446 patients were included in the study between October 2017 and March 2018 and 439 completed the study. Mean (SD) age was 49.5 (14.5) years and mean duration of psoriasis was 14.8 (13.6) years. Over half of the patients (57.2%) were men (Table 1).
Demographic and Clinical Characteristics of Study Population.
| Age, mean (SD), y | 49.5 (14,5) |
| Median | 48.0 |
| Sex, No. (%) | |
| Male | 253 (57.2) |
| Female | 189 (42.8) |
| Duration of psoriasis, mean (SD), y | 14,8 (13.6) |
| Median | 12.0 |
| Episodes in last 6 mo, mean (SD), No. (%) | 2.8 (2.0) |
| Median | 2.0 |
| Previous treatments, No. (%) | |
| Systemic | 23 (3.7) |
| Phototherapy | 12 (2.0) |
| Topical | 577 (94.3) |
| Topical corticosteroids | 348 (60.3) |
| Fixed Cal/BD combination | 189 (32.8) |
| Topical immunosuppressants | 13 (2.3) |
| Other topical treatments | 18 (3.1) |
| Emollients | 9 (1.6) |
| Treatment of most recent flare, No. (%) | |
| Systemic | 16 (4.2) |
| Phototherapy | 2 (0.5) |
| Topical | 359 (95.2) |
| Topical corticosteroids | 145 (40.4) |
| Fixed Cal/BD combination | 118 (32.9) |
| Topical immunosuppressant | 4 (1.1) |
| Another topical treatment | 89 (24.8) |
| Emollient | 3 (0.8) |
| Main comorbidities, No. (%) | |
| Hypertension | 69 (15.5) |
| Dyslipidemia | 52 (11.7) |
| Diabetes | 28 (6.3) |
| Mental and behavior disorder | 25 (5.6) |
| Digestive tract disease | 24 (5.4) |
| Obesity | 19 (4.3) |
| Other skin disorders | 17 (3.8) |
| Heart disorder | 16 (3.6) |
| Arthritis | 16 (3.6) |
Abbreviation: Cal/BD, calcipotriol and betamethasone dipropionate.
In the 6 months leading up to treatment with Cal/BD aerosol foam, 94.3% of the patients had been treated with other topical products, 3.7% had received systemic therapy, and 2.0% had undergone phototherapy. Topical corticosteroids were the most common topical treatment (60.3%). The treatments used to treat the most recent psoriasis flare were topical products in 95.2% of cases, systemic therapy in 4.2%, and phototherapy in 0.5%. The most common comorbidities were hypertension (15.5%), dyslipidemia (11.7%), and diabetes (6.3%) (Table 1).
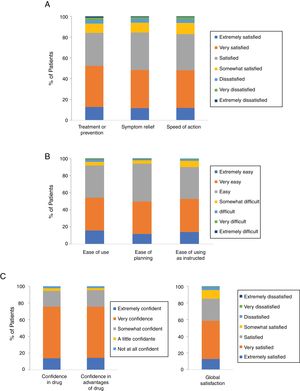
Patient Satisfaction With Cal/BD Aerosol FoamAccording to the results for the effectiveness domain of the TSQM-9, 84% of the patients were satisfied (37.1%), very satisfied (39.6%), or extremely satisfied (12.7%) with the ability of Cal/BD aerosol foam to prevent or treat their psoriasis. Similar results were observed for the foam's ability to relieve their psoriasis symptoms, with 36.1% stating they were satisfied, 36.7% very satisfied, and 11.6% extremely satisfied (84.4% in total). Finally, 82.8% of the patients were satisfied (34.8%), very satisfied (36.2%), or extremely satisfied (11.8%) with the foam's rapid onset of action (Figure 1A).
Most patients also gave positive ratings to the 3 items in the convenience domain; 91.8% thought Cal/BD aerosol foam was easy or very easy to apply, 93.9% though it was easy to plan when they were going to use the foam, and 89.9% thought that it was convenient to use as instructed by their physician (Figure 1B).
High levels of confidence and global satisfaction were also observed; 75.3% of patients stated that they were extremely or very confident that using Cal/BD aerosol foam was good for them and 75.4% were extremely or very certain that the advantages of the treatment outweighed its disadvantages. Finally for the final item in this domain, global satisfaction, 85% of the patients said that were satisfied (26.5%), very satisfied (45.8%) or extremely satisfied (12.7%) (Figure 1C).
Physician Satisfaction With Cal/BD Aerosol FoamEighty-eight physicians rated their satisfaction with Cal/BD aerosol foam as a treatment for the patients recruited on the 5-point Likert scale. Their answers were based on the patients’ clinical histories, their responses to the TSQM-9, changes to psoriasis severity following treatment, and experiences with previous topical treatments.
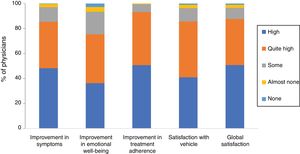
Overall, 85.3% of physicians were of the opinion that Cal/BD aerosol foam had improved their patients’ symptoms a lot (48.2%) or quite a lot (37.1%), and 75.3% thought the same for the effect on emotional well-being. Likely, 93.2% believed that using the foam had a very good (50.6%) or quite good (42.6%) impact on treatment adherence. Finally, 40.9% of the physicians stated that they were very satisfied with the foam vehicle and global satisfaction was very high (50.7%) or quite high (36.9%) (86.9% overall) (Figure 2).
Physician satisfaction with calcipotriol and betamethasone dipropionate aerosol foam. The figure shows the percentage of responses to the Likert scale grouped by domains: improvements in symptoms, emotional well-being, and treatment adherence, satisfaction with vehicle, and global satisfaction.
A Delphi survey organized by the Spanish Psoriasis Group in 2012 determined that the main factors associated with adherence to topical treatments for psoriasis were effectiveness, convenience of use, frequency of application, and vehicle formulation and organoleptic properties.18 All these factors highlight the important link between satisfaction, treatment adherence and health-related quality of life.18 The aim of this study was to determine how satisfied patients and their physicians were with the use of the novel Cal/BD aerosol foam in routine practice in Spain.
The results for the effectiveness domain of the TSQM-9 show that over 80% of patients were satisfied, very satisfied, or extremely satisfied with the ability of Cal/BD aerosol foam to prevent or treat their psoriasis (84%), relieve their symptoms (84.4%), and act fast (82.8%). These positive ratings are consistent with results from previous studies. The advantages of Cal/BD aerosol foam in psoriasis have been demonstrated in several randomized clinical trials that have shown superior results for modified Psoriasis Area and Severity Index scores and treatment success (physician global assessment 0/1).9,12,19 The high satisfaction levels observed for the foam's ability to relieve symptoms is also consistent with findings from other studies that have reported itch relief9,19 and improved overall clinical assessment scores.10 Improvements in itch are closely linked to quality of life, as itching can cause a considerable loss of sleep and productivity.20 The high levels of satisfaction expressed for onset of action are also consistent with previous findings showing that Cal/BD aerosol foam produced significant itch relief by day 3 and a 70% reduction in itch by week 4 in 83.5% of patients.19 The results for the effectiveness domain are also consistent with findings from the PSO-ABLE trial, in which 88.3% of patients perceived Cal/BD aerosol foam as being more effective than previous treatments.12 In brief, the results of our survey on patient satisfaction with Cal/BD aerosol foam support previous findings on satisfaction with treatment effectiveness and are very important considering that effectiveness is the strongest determinant of treatment satisfaction.6,18,21
Perceived convenience is also important as it is closely linked to treatment adherence. According to one study of patient preferences regarding topical vehicles, the most important attributes are a non-greasy or oily texture, ease of application, and a formulation that does not stain.22 Perhaps the high satisfaction levels observed in the convenience domain of our survey are linked to some of these attributes. Our findings regarding convenience and ease of use are consistent with those of an observational study in which 88.6% of patients stated that they found it easy or quite easy to apply the foam.23 In the PSO-ABLE trial, 76.5% of patients also stated that the foam was easy to apply.12 In another study, although similar preference rates were found for Cal/BD gel and aerosol formulations, “immediate feeling of relief” and “felt soothing” were mentioned as benefits of the foam formulation.24
Confidence in Cal/BD aerosol foam was also high in our survey, with a large proportion of patients mentioning that they were very or extremely confident that the treatment would be good for them and that its advantages outweighed its disadvantages. The group as a whole also expressed high levels of global satisfaction. These findings are consistent with the high satisfaction rates observed in the effectiveness and convenience domains and probably indicate increased adherence rates.
In general, our findings related to satisfaction with Cal/BD aerosol foam treatment in routine practice support findings from clinical trials such as PSO-ABLE, which showed that 83.7% of patients preferred Cal/BD aerosol foam to previous topical treatments, as it was perceived as more effective (88.3%) and easier to apply (76.5%) and tolerate (81.0%).12
Good physician-patient communication based on shared decision-making is another important determinant of treatment adherence.25 The goal thus should be to align physician and patient satisfaction. One of the novel aspects of the current study is that we analyzed the opinions of both patients and their physicians. The results from the Likert scale generally show that physicians had similar perceptions to their patients regarding the different attributes of Cal/BD aerosol foam. They were very satisfied with the treatment overall, with the vehicle, and with the product's ability to improve symptoms. These findings coincide with the patients’ scores on the TSQM-9. The most highly rated aspect by physicians was improvement in treatment adherence. As mentioned, this perception is probably linked to the high rates of satisfaction with effectiveness and convenience reported by patients. It is also noteworthy that the physicians also expressed satisfaction with improvements in emotional well-being, as plaque psoriasis is known to have a considerable psychosocial impact.26
The main limitation of this study is that we did not study adherence to treatment or analyze satisfaction levels in relation to duration of Cal/BD treatment or previous treatments.
ConclusionsThis study, the first of its kind, has shown that patients were very satisfied with the effectiveness and convenience of Cal/BD aerosol foam compared with previous treatments. These perceptions are consistent with the positive views of physicians in terms of the product's ability to improve symptoms, emotional well-being, and treatment adherence. These favorable perceptions of the different attributes of Cal/BD aerosol foam could lead to improved adherence in clinical practice.
FundingThis study was funded by LEO Pharma S.A.
Conflicts of InterestManel Velasco has been engaged as a speaker or consultant by Abbvie, Janssen, Novartis, Lilly, Pfizer, and LEO Pharma Silvia Pérez-Barrio has been engaged as a speaker or consultant by Abbvie, Janssen, Novartis, Lilly, LEO Pharma, UCB, Pfizer, and Celgene. The other authors declare no conflicts of interest.
The authors thank Carla Granados from Trialance SCCL for her help with writing this manuscript.
Please cite this article as: Velasco M, González-Fernández D, Rodriguez-Martín M, Sánchez-Regaña M, Pérez-Barrio S. Satisfacción del paciente y el médico con la espuma en aerosol de calcipotriol y dipropionato de betametasona para el tratamiento de la psoriasis vulgar en el cuerpo . https://doi.org/10.1016/j.ad.2019.03.013.