A wide range of treatments are currently available for severe atopic dermatitis, including systemic therapies such as ciclosporin, corticosteroids, azathioprine, methotrexate, mofetil mycophenolate, and omalizumab. In patients who can no longer take systemic drugs or who need a dose reduction, wet-wrap treatment can be an excellent option.
To date, wet wraps have mostly been used in severe cases of childhood atopic dermatitis. We report our experience with wet-wrap treatment in 5 adults with atopic dermatitis and 2 with nodular prurigo. The results were satisfactory and there were few adverse effects.
En la actualidad disponemos de un importante arsenal terapéutico para la dermatitis atópica grave. Entre los tratamientos sistémicos cabe destacar entre otros la ciclosporina, los glucocorticoides, la azatioprina, el metotrexato, el mofetil micofenolato o el omalizumab. La terapia con vendajes húmedos oclusivos (wet-wrap) puede suponer una excelente alternativa en pacientes en los que se pretende evitar o reducir el uso de tratamientos sistémicos.
Hasta el momento los vendajes húmedos se han considerado como una alternativa en los casos de dermatitis atópica grave de la infancia. Aportamos nuestra experiencia en un grupo de 7 pacientes adultos, 5 de ellos con dermatitis atópica y 2 con prurigo nodular, destacando los resultados satisfactorios obtenidos, así como los escasos efectos secundarios observados.
Atopic dermatitis (AD) is a chronic eczematous dermatosis that causes pruritus; patients have a family history of allergic disease and the lesions arise at typical sites. AD causes considerable morbidity, such as difficulty in sleep initiation and maintenance, and also has an emotional impact on patients and their families.1,2
There is an extensive therapeutic arsenal for the topical and systemic treatment of severe AD. The main systemic treatments include antihistamines, ciclosporin, glucocorticoids, azathioprine, methotrexate, mycophenolate mofetil, and omalizumab; in this context, most of these drugs must be prescribed for off-label use and have undesirable effects if treatment is continued for long periods. Wet-wrap treatment is a good alternative in patients in whom the aim is to avoid or reduce the dose of systemic treatments or their complications. To date, reports on the use of this therapy have mainly involved children, and the results have been very good, with few adverse effects; however, experience in adult patients is lacking.3–5
Wet-wrap treatment has been reported to be effective both in AD and in generalized dermatoses that cause pruritus, xerosis, and discomfort, such as nodular prurigo, psoriasis, and the erythrodermas.1
Case DescriptionsWe present our experience in 7 patients (6 men and 1 woman) aged between 16 and 80 years. The diagnoses were severe AD in 5 patients and nodular prurigo in 2; the conditions were resistant to conventional treatments. Wet-wrap was used in 6 patients in monotherapy and 1 patient was also administered daily pulses of 500mgof methylprednisolone for 3 days. In all cases, the wet-wrap treatment was performed during a hospital admission.
The product applied was a mixture of a dilute topical corticosteroid (0.05% fluticasone) and an emollient (petrolatum-cetomacrogol).3,6 All patients were treated for 7 days, with daily changes of the bandages.
The application protocol was as follows: washing of the skin in the morning with warm water and mild soap. The dilute formulation of 0.05% fluticasone propionate, prepared in the hospital pharmacy, was then applied. The extemporaneous drug formulation consisted of 1 part of 0.05% fluticasone propionate cream in 9 parts of petrolatum with 20% cetomacrogol; the formulation was applied in the direction of hair growth. Between approximately 15 and 30g of this formulation (depending on the body surface area treated) was applied to each patient each day.
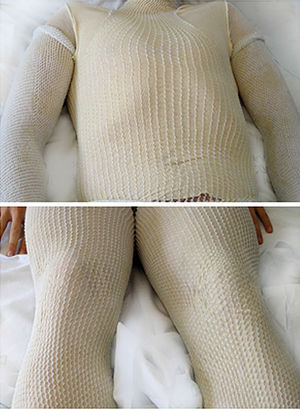
The following step consisted of cutting the tubular bandages (cotton wool-Tubifast) to fit the arms, legs, and trunk and moistening them in warm water. After moistening, these bandages were applied as the first layer of the wet-wrap. A second layer of dry tubular bandages was then fitted over the previous layer (Fig. 1). Every 2 to 3hours the outer bandages must be removed in order to moisten the inner bandages using a warm-water spray. The solution should not be applied at night in order to allow the patient to sleep. This whole procedure is repeated daily for 7 days. Topical calcineurin inhibitors (tacrolimus, pimecrolimus) and topical low-strength corticosteroids were used on the face.
None of the patients in our series developed any infectious skin disease (molluscum contagiosum, viral warts, impetigo, herpesvirus infection, or folliculitis) that would have required interruption of the treatment.
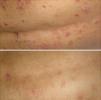
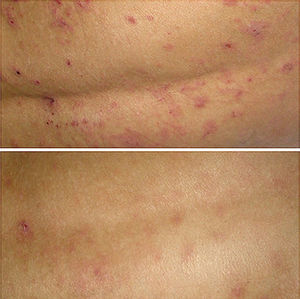
The result on removal of the wet-wrap was a clinical improvement of the lesions in all cases (Fig. 2), with a marked reduction of pruritus and improvement in the health-related quality of life assessed using the SCORing Atopic Dermatitis (SCORAD) index (Table 1). No adverse effects were observed except for a degree of discomfort due to the moisture of the internal bandages.
Variables Studied in Each Case and the Results Obtained on the SCORAD Index.
| Case | Sex | Age | Diagnosis | Before Treatment | After Treatment | Previous Treatments | Other Treatments During Hospitalization |
| P1 | M | 69 | Atopic dermatitis | SCORAD 61 | SCORAD 16 | Oral and topical CS | No |
| P2 | F | 62 | Nodular prurigo | VAS 10 | VAS 6 | Oral and topical CS, ciclosporin | No |
| P3 | M | 21 | Atopic dermatitis | SCORAD 53 | SCORAD 14 | Oral and topical CS, ciclosporin, ustekinumab, UV-B | No |
| P4 | M | 16 | Atopic dermatitis | SCORAD 69 | SCORAD 10 | Oral and topical CS | No |
| P5 | M | 29 | Atopic dermatitis | SCORAD 71 | SCORAD 25 | Oral and topical CS, ciclosporin, omalizumab, MTX, UV-B | No |
| P6 | M | 22 | Atopic dermatitis | SCORAD 66 | SCORAD 11 | Oral and topical CS, mycophenolate mofetil, UV-B, efalizumab | Pulses of methylprednisolone |
| P7 | M | 80 | Nodular prurigo | VAS 9 | VAS 5 | Oral and topical CS, ciclosporin | No |
Abbreviations: CS, corticosteroids; F, female; M, male; MTX, methotrexate; P, patient; SCORAD, SCORing Atopic Dermatitis; VAS, visual analogue scale.
It is important to note that no washout period was used between treatments in the cases presented in our study and the therapy was not repeated in any of the patients. It is difficult to determine the period of remission that the wet-wraps achieved in our patients as all of them subsequently received systemic treatment, which interfered with the interpretation of results. However, this treatment produced an undeniable symptomatic improvement in all patients and achieved temporary remission of up to 30 days, increasing the efficacy of systemic treatments in previously refractory cases.
DiscussionWet-wrap or moist bandaging is defined as a treatment modality based on the use of a double layer of tubular bandages, the inner layer, closer to the skin, being moist; this can be combined with the application of emollient creams or creams containing active substances. The external layer of bandages is dry.
This classic therapeutic modality was introduced in the United Kingdom in 1970 for the treatment of AD in children.6 It subsequently started to be used in Germany, mainly in Munich and Hamburg. Currently, the center with greatest experience in the management of these bandages is the Sofia Pediatric Hospital in Rotterdam, Holland.3 Several studies have been published on the use of these bandages. Dabade et al.,7 in the Mayo Hospital in Minnesota, USA, achieved excellent results with this therapy in 218 patients with severe AD, all under 18 years of age. Improvement was observed in all the patients, and 93% of them presented an improvement of greater than 50%. Another example is the study performed by Bingham et al.,1 also in the Mayo Hospital in Minnesota. Those authors treated 331 patients aged between 15 and 95 years. Interestingly, most of the patients in that latter study were adults with diagnoses that included inflammatory pruritic dermatoses such as AD, erythroderma, psoriasis, nodular prurigo, pityriasis rubra pilaris, dermatomyositis, and mycosis fungoides. The results in that study were also good, with improvement on the first day of treatment in 93.6%; only 1.7% reported no improvement in the pruritus during wet-wrap treatment.
In recent years, wet-wrap treatment has come to be considered a safe and effective technique for severe childhood dermatitis.8 This modality has also been used successfully in other indications, such as guttate psoriasis, psoriatic erythroderma, treatment-resistant pruritus, active urticaria pigmentosa, lamellar ichthyosis, and T-cell lymphoma.1,6 The technique may be effective in all dermatoses associated with pruritus, inflammation, and general discomfort.
The mechanism of action that explains its efficacy is a recovery of the epidermal barrier by increasing the water content of the epidermis, decreasing transepidermal water loss, increasing the release of lamellar bodies, and restoring the laminar structure of the intercellular lipids.5 Furthermore, this technique protects the skin from scratching and reduces pruritus and, through vasoconstriction secondary to cooling of the skin due to evaporation of the moisture, it reduces inflammation.7 In addition, the absorption of topical corticosteroids is increased. Healing is further improved by the moist environment protected from external allergens. All this leads to a marked clinical improvement, evidenced by a significant reduction in the SCORAD index.
The adverse effects of wet-wrap treatment are few and are not particularly serious. The most common is discomfort due to the limitation of mobility caused by the bandages, and shivering due to the moist inner bandages; this can be avoided by controlling the temperature of the water that is applied.3 Other possible adverse effects are folliculitis secondary to the occlusive effect of the bandages; the risk can be reduced by applying the product in the direction of hair growth. Other more serious complications are skin infections due to Pseudomonas spp.; these are rare but can be favored by the moist environment created by these bandages. Cases of impetigo and herpesvirus infections have also been reported, and there are studies such as the one by Hindley et al.9 that have demonstrated a higher incidence of these infections compared with nonocclusive therapies. However, it should be remembered that these infections are well-known problems associated with AD in childhood and are easily managed. If skin infection does occur, the wet-wrap therapy should be temporarily interrupted and appropriate treatment of the infection administered. Some authors have also considered there to be a relative contraindication to the use of these bandages in adolescents during puberty due to the risk of striae.3,6 We should also add that there are isolated case reports of suppression of the hypothalamic-hypophyseal-adrenal axis, with a fall in morning blood cortisol levels; this resolves within 2 weeks after treatment withdrawal.1,8 Given the low strength and concentration of the corticosteroid used, the treatment duration of less than 2 weeks, and the low possibility of absorption of the topical fluticasone, we did not measure the blood cortisol levels in our patients.
In conclusion, we consider that wet-wrap therapy constitutes a safe and effective method for the treatment of severe or refractory AD in children and adults. Its advantages include a rapid response, reduced pruritus, and an improvement in the quality of sleep, as well as a reduction in the need for potentially dangerous systemic treatments. There is also a possibility for home treatment if the patients and family are adequately trained. The disadvantages of this method include the high cost, the need for trained staff, variable tolerance, and the adverse effects mentioned above.6,7
For all these reasons, we believe wet-wrap therapy should be included as an option in the management of severe or refractory AD and other inflammatory dermatoses, both in children and in adults. Its main benefit is the rapid control of a flare-up that will enable us to consider new maintenance therapies.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Albarrán-Planelles C, Jiménez-Gallo D, Linares-Barrios M, Martínez-Rodríguez A. Vendajes húmedos: nuestra experiencia. Actas Dermosifiliogr. 2014;105:e18–e21.