Approaches to treating melanoma have changed radically since the introduction of immunotherapy, and survival figures are now higher than possible with earlier therapies. The immunomodulators currently available mainly block CTLA-4 (cytotoxicT lymphocyte-associated molecule-4) and PD-1 (programed cell death protein 1) translocated to the cell surface, where they inhibit the antitumor immune response. Treatments blocking these molecules are being more widely used. Research now seeks new molecular targets, the best combinations of available drugs, and biomarkers that can identify ideal candidates for each one
Desde la introducción de la inmunoterapia el tratamiento del melanoma ha sufrido una revolución, consiguiéndose cifras de supervivencia superiores a las que se alcanzaban con los tratamientos previos. Los fármacos inmunomoduladores disponibles actualmente van dirigidos principalmente frente a las moléculas de superficie CTLA-4 y PD-1, que ejercen un efecto inhibidor sobre la respuesta inmune antitumoral. Se trata de un tratamiento en pleno proceso de expansión, y la investigación se dirige hacia el descubrimiento de nuevas moléculas, las combinaciones de los fármacos disponibles o la identificación de biomarcadores que permitan seleccionar a los pacientes idóneos para cada terapia.
Melanoma is the eighth most common cancer. It accounts for approximately 5% of all new cancer cases, and incidence is rising steadily.1,2 The incidence of cutaneous melanoma in the Spanish population is 8.7 cases per 100 000 population.3 Survival rates for early-stage melanoma are higher than 95%, but prognosis worsens considerably for disseminated forms, which have a 5-year-survival rate of less than 20%.2 Chemotherapy is the most widely accepted treatment for advanced cutaneous melanoma, but response rates are lower than 15% and the impact on survival outcomes is minimal.4
The emergence of immunotherapy marked an important change in the search for alternative treatments. The association between cancer cells and the immune system has been recognized for decades, and cutaneous melanoma is probably one of the most immunogenic tumors that exist. The interaction between the immune system and melanoma is dynamic, however, and tumor cells use various mechanisms to avoid recognition and destruction by the immune system, favoring disease progression.5
Treatment strategies aimed at boosting the immune response through vaccines and biochemotherapy were attempted for several years, but the results were disappointing.6,7 A more recent approach, however, that of using monoclonal antibodies directed against immune checkpoint inhibitors, has been met with enthusiasm.8
Evidence Supporting the Immune Response in MelanomaIn contrast to other cancers, numerous findings, both clinical and histological, support the involvement of the immune system in the fight against melanoma.9
An inflammatory infiltrate within and around the tumor, sometimes accompanied by tumor regression, is observed in the majority of melanomas. Signs of regression are observed in up to 35% of cases, and tumor regression is actually 6 times more common in melanoma than in other types of tumors.10
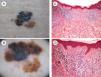
Another more recently described finding is a correlation between the density of high endothelial venules and lymphocytic infiltration in association with greater tumor regression.11 An association has also been observed between tumor regression and melanomas with a lower mitotic or proliferative rate, i.e., melanomas with a better prognosis12 (Fig. 1).
Image showing regression in melanoma. A, Clinical image showing a central region with loss of pigment. B, Dermoscopic features of the lesion showing a multicomponent pattern with a central white area. C and D, Histologic images showing a dense lymphocytic infiltrate in the dermis without epidermal involvement, together with melanophages and fibrosis in the dermis (hematoxylin-eosin, original magnification ×10 [C] and ×20 [D]).
The appearance of a halo around nevi provides further evidence of autoimmune reactivity and this halo can eventually cause the nevus to disappear completely. Vitiligo has also been observed in 2% to 7% of advanced melanomas following systemic therapy and has been linked to a better prognosis.13
Although the above findings and observations only serve to generate hypotheses, numerous studies have demonstrated the participation of the immune system in regressive melanoma. Findings include predominant infiltration of tumors by T lymphocytes, which are mediators of the innate immune system,14 and oligoclonal expansion of T lymphocytes in melanomas with extensive areas of regression.15 Adoptive cell therapy with T lymphocytes isolated from regressive melanomas has also been show to generate cytolytic activity against autologous melanomas.16
Whatever the case, the development of melanoma implies a breakdown in the body's defense system. This immune system failure is mediated by both factors that are intrinsic to the tumor cells and impaired immune defenses, which reduce the ability of the immune system to exert its effector function.
Suboptimal activation of specific T lymphocytes due to weak or absent expression of antigens in melanoma cells is known to result in immune response failure.17 The expression of endothelial adhesion molecules in the tumor is also reduced, and in addition, certain cells in the tumor infiltrate, such as macrophages, release growth factors, cytokines, and suppressors of immune response.18,19
The antitumor immune response alone thus is not sufficient to produce the elimination of tumor cells, but it can favor response to immunotherapy.
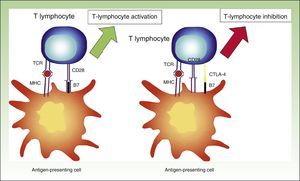
Antigens That Trigger an Immune ResponseTwo signals are needed for the activation of T lymphocytes. First, the T-cell receptor (TCR) recognizes the antigen presented by dendritic cells through the major histocompatibility complex, and second, the cluster of differentiation (CD) antigen 28 expressed on the surface of T lymphocytes produces a costimulatory signal by binding to its ligands B7-1 or B7-2. Without this second signal, the T lymphocytes would not respond to the stimulus, resulting in anergy.20
Activated T lymphocytes must then migrate to the site of the tumor, where they will act to destroy the tumor cells.21 These intratumoral cells, however, often exhibit decreased proliferation, cytokine production, and cytolytic activity, although outside the tumor microenvironment, they have been shown to mount powerful specific responses, suggesting the existence of signals in this microenvironment that block lymphocyte function.22
The activation of TCR also produces signals that stimulate immune checkpoint inhibitors. Cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are cell surface receptors involved in the regulation of activated T lymphocytes. They belong to the B7-CD28 superfamily and by binding to their ligands, they trigger inhibitory pathways resulting in reduced T-lymphocyte activity.23
Cytotoxic T-Lymphocyte Associated AntigenActivated T lymphocytes express CTLA-4. This receptor is expressed approximately 24 to 48hours after activation and it binds to B7 with a greater infinity than to CD28, providing an inhibitory signal to T lymphocytes.24
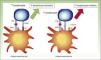
CTLA-4 is a powerful coinhibitor that has a role in the development of tolerance from early on. Studies of CTLA-1-/− mice have shown that the absence of CTLA-4 results in a massive infiltrate of autoreactive T lymphocytes in diverse tissues, with death occurring within 2 to 3 weeks25,26 (Fig. 2).
Diagram showing T lymphocyte activation. Activation of T lymphocyte after interaction between TCR and HLA and between costimulatory molecules B7 and CD28 (left). Inhibition of response following binding of CTLA-4 to B7 (right).
TCR indicates T-cell receptor; CD28, cluster of differentiation 28; MHC, major histocompatibility complex; CTLA-4, cytoxic T-lymphocyte associated protein 4.
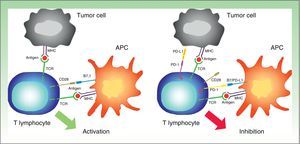
PD-1 is a transmembrane receptor of the CD28 family that is normally expressed in activated CD4+ and CD8+ lymphocytes.27 It has an immunosuppressive function, which under normal conditions prevents an excessive immune response to autologous cells. PD-1 expression is induced by the activation of T lymphocytes and is interrupted when the antigen is eliminated by the immune response. If this response is inadequate, however, PD-1 expression persists, generating a phenotype of ineffective T lymphocytes.28
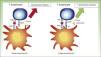
PD-1 has 2 ligands: PD-ligand 1 (PD-L1), also known as B7-H1 and CD274, and PD-L2 (B7-DC or CD273). PD-L1 is expressed in more cells than PD-L2. When PD-L1 is expressed on the surface of tumor cells and binds to PD-1 on T lymphocytes, it activates a signaling cascade that suppresses TCR activation, thereby preventing the release of growth factors and survival signals.29 Evidently, thus, binding of PD-1 expressed on the surface of T lymphocytes to its ligand has a key role in blocking the immune response (Fig. 3).
Diagram showing PD-1 and PD-L1 activity. Activation of T lymphocyte by interaction between stimulatory and costimulatory molecules (left). Inhibition of T cell following binding of PD-1 to PD-L1 (right).
APC indicates antigen-presenting cell; MHC, major histocompatibility complex; TCR, T-cell receptor; CD28, cluster of differentiation 28; PD-1, programmed death protein 1; PD-L1, programmed death protein ligand 1.
Numerous treatment strategies aimed at boosting the antitumor immune response have been developed since the 1980s, but they have had limited success.16 Recent years, however, have witnessed the emergence of a new strategy based on the blockage of receptors that inhibit the antitumor immune response rather than on the direct reactivation of the immune system. The antibodies used in these treatments bind not to the tumor cells but to the lymphocytes in order to stimulate a response.
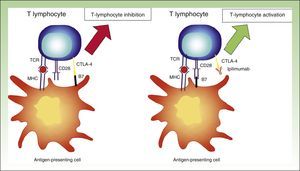
CTLA-4 InhibitionT lymphocytes express CTLA-4 on their surface within 2 to 3 days of activation and as a consequence exhibit reduced activity.30 In theory, thus, CTLA-4 blockade would allow specific T cells to maintain their activity.
The above theory was proven by the use of fully humanized monoclonal antibodies targeting CTLA-4, such as ipilimumab. By interfering with CTLA-4-B7 binding, the inhibitory stimulus disappears, and the lymphocytes maintain their activity.31 While this interaction means that the immune system will continue to fight against the tumor cells, it also means that it will target nontumor cells (Fig. 4).
Diagram showing activity of ipilimumab. T-lymphocyte inhibition through binding between CTLA-4 and B7 (left). Activation of T lymphocyte through CTLA-4 blockade following binding between CTLA-4 and the monoclonal antibody ipilimumab.
TCR indicates T-cell receptor; CD28, cluster of differentiation 28; MHC, major histocompatibility complex; CTLA-4, cytoxic T-lymphocyte associated protein 4.
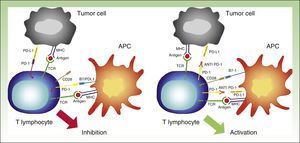
PD-1 needs to bind to its ligands in order to exert its inhibitory effect. Most tissues, including tumor cells, express PD-L1. PD-L2, by contrast, is expressed only on dendritic cells, macrophages, mast cells, and B lymphocytes.32 PD-L1 expression on tumor cells can be mediated by oncogenic processes secondary to activating mutations, or activated by interferon released by specific T lymphocytes infiltrating the tumor.33,34 PD-L1 expression, however, can apparently only be induced if T lymphocytes expressing PD-1 exist. This phenomenon is known as adaptive immune resistance35 (Fig. 5).
Interaction between PD-1 and PD-L1 leading to T-lymphocyte inhibition (left). Binding between anti PD-1 drugs such as nivolumab or pembrolizumab to PD-1 on the surface of the lymphocytes, resulting in their activation and hence the generation of the antitumor response (right).
APC indicates antigen-presenting cell; MHC, major histocompatibility complex; TCR, T-cell receptor; CD28, cluster of differentiation 28; PD-1, programmed death protein 1; PD-L1, programmed death protein ligand 1.
The above pathway can be inhibited by blocking PD-1, preventing it from binding to PD-L1 or PD-L2, or blocking PD-L1, impeding thus its interaction with PD-1.
Unlike CTLA-4-deficient mice, PD-1- and PD-L1-deficient mice do not die early, but rather progressively develop multiple autoimmune disorders.36,37 This would explain the wide range of adverse effects observed in patients treated with immunomodulators.
Oncolytic VirusesOncolytic viral therapy is a new tool that has proven effective in the treatment of multiple solid tumors, including melanoma.38 The best results to date have been obtained with oncolytic herpes simplex virus type 1 (HSV-1) vectors. HSV-1 is a strongly oncolytic genetically modified virus that selectively infects and replicates in tumor cells, but spares healthy cells.39
Immunomodulatory DrugsAnti-CTLA-4 AntibodiesIpilimumabIpilimumab was the first immunomodulatory drug with proven survival benefits in patients with metastatic melanoma.31,40 It was approved by the US Food and Drug Administration (FDA) in 2011 and by the European Medicines Agency (EMA) just a few months later41 (Table 1).
Commercial Data, Characteristics, and Administration Route for Currently Available Immunomodulatory Drugs.
| Agent | Previous Names | Commercial Name | Pharmaceutical Company | Therapeutic Target | Type of Molecule | Administration Route | Dose |
|---|---|---|---|---|---|---|---|
| Ipilimumab | MDX-010 MDX-101 | Yervoy | Bristol-Myers Squibb | CTLA-4 | Humanized recombinant IgG1 antibody | Intravenous | 3mg/kg every 3 wk up to 4 doses |
| Nivolumab | MDX-1106, ONO-4538 BMS-936558 | Opdivo | Bristol-Myers Squibb | PD-1 | Fully humanized IgG4 antibody | Intravenous (for 60min) | 3mg/kg every 2 wk |
| Pembrolizumab | MK-3475, Lambrolizumab | Keytruda | Merck | PD-1 | Humanized IgG4 antibody | Intravenous | 2mg/kg every 3 wk |
| Pidilizumab | CT-011 | – | Medivation | PD-1 | Humanized IgG1 antibody | Intravenous | 1.5mg/kg |
| Atezolizumab | MPDL-3280A | – | Roche | PD-L1 | Humanized IgG1 antibody | Intravenous | 15mg/kg every 3 wk |
| Talimogene laherparepvec | JS1/34.5-/47-/(GM-CSF); Onco-VexGM-CSF | Imlygic | Amgen | Tumor cells | Attenuated HSV-1 | Subcutaneous | First visit: Vial containing 106 PFU/mL Subsequent visits: Vial containing 108 PFU/mL (maximum 4mL/visit) |
Abbreviations: CTLA-4, cytoxic T-lymphocyte associated protein 4; HSV-1, herpes simplex virus type 1; Ig, immunoglobulin; PD-1, programmed death protein 1; PFU, plaque-forming units; PD-L1, programmed death protein ligand 1
A randomized controlled trial comparing ipilimumab and dacarbazine showed longer overall survival for patients treated with ipilimumab.42 Median survival was just 9.5 months, but what was remarkable was that 22% of patients survived long term.43
Because of its mechanism of action, ipilimumab is associated with a large number of adverse effects, most of which are of an autoimmune nature. Adverse effects have been reported in approximately 60% of patients treated with this drug.44 The most common effects are diarrhea, colitis, dermatitis, hypophysitis, and thyroiditis. Just 10% to 15% of adverse effects are moderate or severe (grade 3 or 4) and they tend to resolve within 4 to 6 weeks with corticosteroids or in a relatively short time with other immunosuppressants45 (Table 2).
Classification of Adverse Effects Associated With Pharmacological Treatment Based on the Common Terminology Criteria for Adverse Events (Version 4.0) Issued by the National Cancer Institute.
| Grade | Category | Description |
|---|---|---|
| I | Mild | Asymptomatic or mild symptoms. Intervention not indicated. |
| II | Moderate | Symptomatic, limits instrumental activities of daily living.a Local or noninvasive intervention indicated. |
| III | Severe | Not immediately life-threatening but limits self-care activities of daily living.b Hospitalization indicated. |
| IV | Very Severe | Life-threatening adverse effects. Urgent intervention indicated. |
| V | Death | Death related to administration of drug. |
Source: National Cancer Institute.45
Pseudoprogression is a common phenomenon following the introduction of immunomodulatory drugs. It is due to the activation of T lymphocytes and the release of cytokines, which produce chemotaxis of inflammatory mediators, edema, and even local necrosis, causing a temporary increase in tumor volume.44 Pseudoprogression is more common in melanoma than in other tumors, and it is something that clinicians need to be familiar with in order not to interrupt treatment in patients who could ultimately benefit from immunotherapy, despite the initial increase in tumor size.
Pseudoprogression is also a limiting factor for the use of immunomodulatory drugs in patients with advanced metastatic disease in high-risk locations, such as metastases to the central nervous system. An increase in tumor volume, albeit temporary, would be life-threatening in such cases.
TremelimumabTremelimumab is an IgG2 anti-CTLA-4 antibody. Despite the successful results reported in early-phase trials, the phase III trial showed no significant differences between tremelimumab and chemotherapy, and the drug was therefore not approved for use in advanced melanoma.46
Anti-PD-1 AntibodiesNivolumabNivolumab was the first anti-PD-1 antibody that showed good tolerance and significant response in several patients with solid tumors.47 It was approved for use in Spain at the end of 2015, and in February of the following year, was made available for patients with unresectable or metastatic melanoma, regardless of BRAF mutation status. It is a monoclonal antibody with high affinity for PD-1. It blocks interactions between this receptor and PD-L1 and PD-L2, increasing specific T lymphocyte proliferation and cytokine production.47,48
The results of the first phase III trial showed nivolumab to be superior to chemotherapy, and this superiority was confirmed in later trials48,49 (Table 3). The main treatment-related adverse effects are fatigue, diarrhea, nausea, anorexia, and fever. Practically all the adverse effects resolve spontaneously or with the administration of systemic corticosteroids. Autoimmune-type effects include cutaneous effects (exanthema, pruritus, vitiligo), gastrointestinal effects (diarrhea, colitis), elevated liver enzymes, hypothyroidism or hyperthyroidism, and pneumonitis.51 Although adverse effects are relatively uncommon, careful monitoring of patients, together with initiation of treatment where necessary, is essential.
Diagram Summarizing Some of the Most Significant Clinical Trials and Their Results for Response Rates, Survival, and Adverse Effects.
| Studya | Drug | Phase | Population | Patients, No. | Arms | Response Rates | DFS, mo | Adverse Effects (Grade ≥3) |
|---|---|---|---|---|---|---|---|---|
| Hodi et al. (2010)40 | Ipilimumab | III | Previously treated advanced melanoma | 676 | Ipilimumab+vaccine gp100 Ipilimumab Vaccine gp100 | Combination: 5% Ipilimumab: 10.9% Vaccine gp100: 1.5% | DFS combination: 10 DFS ipilimumab: 10.1 DFS vaccine gp100: 6.4 | Ipilimumab and combination: 10%-15% Vaccine gp100: 3% |
| Topalian et al. (2012)48 | Nivolumab | I | Advanced melanoma | 104 | Nivolumab as monotherapy | 28% | DFS>11.1 | 14% |
| Robert et al. (2015)49 | Nivolumab | III | Previously untreated advanced melanoma BRAF-WT | 418 | Nivolumab DCZ | Nivolumab: 40% DCZ: 13.9% | DFS: 5.1 | Nivolumab: 11.7% DCZ: 17.6% |
| Weber et al. (2015)50 [Checkmate-037] | Nivolumab | III | Advanced melanoma after ipilimumab or anti-BRAF | 405 | Nivolumab QT DCZ Paclitaxel | Nivolumab: 31.7% QT: 10.6% | DFS Nivolumab: 4.7 DFS QT: 4.2 | Nivolumab: 9% QT: 31% |
| Hamid et al. (2013)52 | Pembrolizumab | I | Advanced melanoma | 135 | Pembrolizumab as monotherapy | 38% | DFS>7 | 13% |
| Ribas et al. (2015)55 [Keynote-002] | Pembrolizumab | II | Advanced melanoma in patients resistant to ipilimumab | 540 | Pembrolizumab 0.2mg/kg 0.10mg/kg QT | Pembrolizumab: 21% (25% 10mg/kg) QT: 4% | DFS pembrolizumab: 5.4 (5.8 in 10mg/kg arm) DFS QT: 3.6 | Pembrolizumab: 11% (14% 10mg/kg) QT: 26% |
| Robert et al. (2015)56 [Keynote-006] | Pembrolizumab | III | Advanced melanoma | 834 | Pembrolizumab Ipilimumab | Pembrolizumab: 33% Ipilimumab: 12% | DFS pembrolizumab: 5.5 DFS ipilimumab: 2.8 | Pembrolizumab: 10% Ipilimumab: 20% |
| Andtbacka et al. (2015)64 | T-VEC | III | Advanced melanoma | 436 | Intralesional T-VEC Subcutaneous GM-CSF | T-VEC: 26% GM-CSF: 5.7 | DSF T-VEC: 8.2 DFS GM-CSF: 2.9 | T-VEC: 36% (2% grade>3) GM-CSF: 21% |
| Wolchok et al. (2013)67 | Ipilimumab+nivolumab | I | Advanced melanoma | 53 | Ipilimumab+nivolumab Sequential treatment | Combination: 40% Sequential: 20% | DFS combination >5 DFS sequential >2 | Combination: 53% Sequential: 18% |
| Postow et al. (2015)68 | Ipilimumab+nivolumab | I | Previously untreated advanced melanoma | 142 | Ipilimumab+nivolumab Ipilimumab only | Combination: 60% (22% complete response) Ipilimumab: 11% (partial response) | DFS combination>11 (study cutoff) BRAF-WT/8.5 BRAF+ DFS ipilimumab: 4.4 for BRAF-WT/2.7 for BRAF+ | Combination: 54% Ipilimumab: 24% |
| Larkin et al. (2015)69 | Ipilimumab+nivolumab | III | Previously untreated advanced melanoma | 945 | Ipilimumab+nivolumab Ipilimumab Nivolumab | Combination: 57.6% Ipilimumab: 19% Nivolumab: 43.7% | DFS combination: 11.9 DFS ipilimumab: 2.9 DFS Nivolumab: 6.9 | Combination: 55% Ipilimumab: 27.3% Nivolumab: 16.3% |
Abbreviations: BRAF+, patients with BRAF mutation; BRAF-WT, patients without BRAF mutation (wild-type); DCZ, dacarbazine; DFS, disease-free survival; T-VEC, talimogene laherparepvec; QT, chemotherapy.
Pembrolizumab is the monoclonal antibody with the highest affinity for PD-1. The results from phase I and II trials led the authorities to grant accelerated approval for the use of this drug in patients with advanced melanoma who did not respond to ipilimumab.52,53 Pembrolizumab is associated with better responses and survival rates than either chemotherapy or ipilimumab.54–56 The most common adverse effects are asthenia, skin rash, pruritus, and diarrhea52,55 (Table 3).
Both nivolumab and pembrolizumab were approved for use in advanced (unresectable or metastatic) melanoma by the Spanish Agency for Medicines and Medical Devices (AEMPS) in January 2016.57,58
Another anti-PD-1 antibody, pidilizumab, was found to be inferior to both nivolumab and pembrolizumab and was not granted approval.59
Anti PD-L1 AntibodiesAtezolizumabThe first results reported for atezolizumab in melanoma showed response rates of up to 29%. When combined with vemurafenib, however, an objective response rate (for complete and partial responses) of 76% was observed.60
BMS-936559BMS-936559 is a fully humanized IgG4 anti-PD-L1 antibody. Preliminary studies have shown response rates of over 17% and a low rate of adverse effects (9%).61
MED14736MED14736 is a fully humanized IgG1 anti-PD-L1 antibody. It is currently being investigated in a combined study together with dabrafenib and trametinib. Provisional results have shown response rates of over 65% and severe adverse effects in 35% to 40% of patients.62
Oncolytic VirusesTalimogene LaherparepvecTalimogene laherparepvec was approved for use in patients with unresectable melanoma and regional and distance metastases (stages IIIB, IIIC, and IVM1a) without visceral involvement at the end of 2015.63 It is an attenuated HSV-I vector produced by genetic recombination, designed to specifically replicate inside tumor cells and produce human granulocyte-macrophage colony stimulating factor (GM-CSF) with the aim of stimulating the systemic antitumor response.
It is administered subcutaneously and must be injected directly into cutaneous melanoma lesions. In patients with multiple lesions, the largest lesions must be treated first
Treatment with talimogene laherparepvec has shown response rates of 26%, together a favorable adverse effect profile and increased in long-term survival.64 Compared with ipilimumab and vemurafenib, talimogene laherparepvec showed comparable or slightly superior overall survival outcomes, particularly in patients with visceral metastasis.65
Drug CombinationsCTLA-4 and PD-1 exert an inhibitory function through different pathways, and therefore blockade of both receptors should favor the destruction of tumor cells by the immune system. This theory was first proven in animal models66 and later in humans. To date, randomized clinical trials have investigated the combined use of nivolumab and ipilimumab and shown higher response rates than those achieved with either treatment alone.67–69 Nonetheless, over half of the patients treated developed serious adverse effects. The most common serious effects were colitis, diarrhea, and elevated liver enzymes, and most cases required treatment with systemic corticosteroids.68,69
The combined use of nivolumab and ipilimumab was approved by the FDA in September 2015 and is currently pending approval by the EMA.
Biomarkers of Response to ImmunotherapyFollowing the success of immunotherapy, research has now turned to the search for biomarkers capable of identifying patients with a greater chance of responding to treatment.
Expression of PD-L1 in pretreatment tumor samples has shown the clearest association with response to date, with an increased response to anti PD-1 and anti PD-L1 antibodies observed in PD-L1-positive patients.34,70,71 No association has been found between PD-L1 expression and response to ipilimumab and nivolumab combination therapy.68
PD-L1 has several disadvantages in terms of its potential as a biomarker. Its expression is highly variable, even in samples from the same patient, and in addition, although greater clinical benefits have been observed in PD-L1-positive patients, a considerable number of PD-L1-negative patients also respond to treatment.60,70
Interferon released by CD8+ cells infiltrating the tumor margin and parenchyma is another potential confounder, as it induces PD-L1 expression in tumor cells (adaptive immune response). PD-L1 expression is thus an indirect marker of antigen-specific T lymphocyte activation. Tumors with higher levels of PD-L1 and PD-1 respond better to treatment, but specific CD8+ lymphocytes must first be present.72
Generally speaking, the best biomarker currently available for predicting response to any immunomodulatory drug is the presence of CD8+ at the tumor margin.72
Future ProspectsFuture combinationsMultiple trials are underway investigating combinations of immunomodulatory drugs and other treatments for use in patients with advanced melanoma.
Both CTLA-4 and PD-1 inhibit immune response through different pathways, and it is therefore essential to consider their synergistic use. In the case of targeted therapies and oncolytic viral therapy, the antigens released as a result of the death of tumor cells are presented to the lymphocytes by antigen-presenting cells, which also release certain cytokines and chemokines. These events all induce an antitumor immune response, and in this sense anti-BRAF drugs, oncolytic viruses, and peptidic vaccines could favor response to immunomodulatory drugs.73
Adjuvant TherapyPreventive treatment in patients with a greater risk of metastasis is one of the key challenges in the management of melanoma. The options currently used to control locally advanced tumors are surgery, sentinel lymph node biopsy, and interferon α. Nonetheless, the possibility of effectively treating these patients with other agents could change the course of disease, as well as management approaches by dermatologists.
In October 2015, the FDA, following the results of a clinical trial, approved ipilimumab for use as adjuvant therapy in patients with locally advanced melanoma, i.e., in patients with stage iii melanoma, lymph node metastases >1mm, and complete lymph node dissection.74 The trial showed a 5-year disease-free survival rate of 40.8% versus 30.3% for the placebo group.75 Nevertheless, 45% of patients experienced grade 3 or 4 adverse effects, although the severity of these effects can be considerably reduced through adequate management. The most common adverse effects associated with ipilimumab are diarrhea, colitis, endocrine effects (hypophysitis with hypopituitarism, hypothyroidism or hyperthyroidism, and adrenal dysfunction), vitiligo, exanthema, pruritus, and fatigue.
Multiple studies are currently investigating the use of immunomodulatory drugs other than ipilimumab as adjuvant therapy for patients with high-risk tumors following complete excision, and their results will become available in the coming years.76
ConclusionsTreatment of melanoma has changed significantly since the introduction of immunotherapy scarcely 5 years ago, and survival rates are much higher than those obtained with previous treatments.
Nevertheless, the discovery of any new treatment necessitates new studies to better understand the underlying mechanisms of action. Research in the field of immunotherapy and melanoma should focus on identifying criteria for selecting the most suitable candidates for treatment and exploring which drugs or combinations of drugs produce the best responses. The effectiveness of immunomodulatory drugs could be even further enhanced by combining their use with other approaches, such as targeted therapy and oncolytic viral therapy.
In conclusion, boosting the immune response is a key factor in the treatment of melanoma and will probably form part of all treatment regimens yet to come.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr Isabel Pinazo and Dr Carlos Monteaguda for their help with this review.
Please cite this article as: Escandell I, Martín JM, Jordá E. Novedades en inmunología del melanoma. Actas Dermosifiliogr. 2017;108:708–720.






![Image showing regression in melanoma. A, Clinical image showing a central region with loss of pigment. B, Dermoscopic features of the lesion showing a multicomponent pattern with a central white area. C and D, Histologic images showing a dense lymphocytic infiltrate in the dermis without epidermal involvement, together with melanophages and fibrosis in the dermis (hematoxylin-eosin, original magnification ×10 [C] and ×20 [D]). Image showing regression in melanoma. A, Clinical image showing a central region with loss of pigment. B, Dermoscopic features of the lesion showing a multicomponent pattern with a central white area. C and D, Histologic images showing a dense lymphocytic infiltrate in the dermis without epidermal involvement, together with melanophages and fibrosis in the dermis (hematoxylin-eosin, original magnification ×10 [C] and ×20 [D]).](https://static.elsevier.es/multimedia/15782190/0000010800000008/v1_201710010015/S1578219017302354/v1_201710010015/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)