Basal cell carcinoma (BCC) is the most prevalent malignant tumor in humans and the local destruction of tissue that can result from excision has a significant impact on well-being. Treating BCC is costly for health care systems given the high incidence of this tumor, especially in older patients. Standard treatment involves either resection with histologic assessment of margins or Mohs micrographic surgery. Surgery is sometimes contraindicated, however, due to the presence of significant comorbidity or high cosmetic expectations. For such patients, nonsurgical treatments have become available. These alternatives can offer good local control of disease, preserve function, and achieve excellent cosmetic results.
El carcinoma basocelular (CBC) es el tumor maligno más frecuente en seres humanos, y tiene la capacidad de causar una significativa morbilidad asociada a su potencial de destrucción local. El tratamiento del CBC demanda altos costes de atención para los sistemas de salud, por la gran incidencia de esta enfermedad, especialmente en pacientes mayores. El tratamiento estándar para la mayoría de los CBC consiste en la resección quirúrgica con márgenes y control histológico de los bordes de sección, o mediante cirugía micrográfica de Mohs. Sin embargo, en algunos pacientes con contraindicación para cirugía, que tienen comorbilidades importantes o altas expectativas estéticas, existen en la actualidad nuevas alternativas terapéuticas no quirúrgicas, con las cuales se puede lograr muy buen control local, preservar la función y obtener un excelente resultado cosmético.
Basal cell carcinoma (BCC) is the most common cancer in humans.1,2 Although it rarely metastasizes, it can cause significant morbidity due to the invasion and destruction of neighboring anatomic structures.3
Australia has the highest incidence of nonmelanoma skin cancer (NMSC) in the world, with an age-standardized rate of 213 cases per 100000 males and 113 cases per 100000 females.4,5 In Spain, the crude incidence of BCC is 113.05 cases per 100000 inhabitants per year (95% CI, 89.03-137.08),6–8 and according to estimates from the United States, over 2 million cases of NMSC were diagnosed in 2013.9,10
The annual cost of treating NMSC in the US population has been estimated at over $420 million,11,12 and according to data published in 2009, over £240 million was spent on NMSC treatment in England in 2002.13,14
Surgery is the established treatment of choice for BCC for 2 main reasons: it offers the highest cure rates and permits histologic control of margins.7 The emergence of nonsurgical alternatives, however, means that excellent oncologic and cosmetic results are now possible in subgroups of patients with BCC with a low risk of recurrence or in whom surgery is contraindicated for medical reasons. The aim of this article was to review the literature on the nonsurgical treatment of BCC.
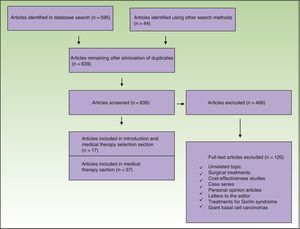
MethodWe performed a literature review to identify articles and clinical practice guidelines discussing the nonsurgical management of BCC that were published in the following databases between 2006 and 2016: MEDLINE via Ovid, EMBASE, Lilacs, and the Cochrane Library. We also performed a hand search.
Studies in which patients had received nonsurgical treatment for BCC were included. We excluded studies in nonhumans, economic evaluations, case series, personal opinion articles, and letters to the editor (Fig. 1).
The initial search retrieved 639 articles, 466 of which were excluded (Fig. 1). The articles that met the inclusion criteria were reviewed separately by 2 authors and any discrepancies were resolved by a third author.
Thirty-seven articles were analyzed for the section on the medical treatment of BCC; 20 of these were reviewed in 4 systematic reviews, 2 of which included a meta-analysis. There were also 7 clinical trials, 6 review articles, and 2 clinical management guidelines evaluating different treatments for BCC. The 17 remaining articles used for this section were evaluated in the systematic reviews included. The methodological quality of the systematic reviews assessed using AMSTAR was high in 3 cases and acceptable in 1 (Table 1). The characteristics of the clinical trials are summarized in Table 2.
Characteristics of the Systematic Reviews and Meta-Analyses Analyzed in Our Review.
| Authors | Title | Year | Type of Study | Level of Methodological Quality Evaluated Using AMSTAR |
|---|---|---|---|---|
| Bath-Hextall et al.39 | Interventions for basal cell carcinoma of the skin | 2007 | Systematic review | High |
| Roozeboom et al.20 | Overall treatment success after treatment of primary superficial basal cell carcinoma: A systematic review and meta-analysis of randomized and nonrandomized trials | 2012 | Systematic review and meta-analysis | High |
| Charlotte et al.12 | Basal cell carcinoma: An evidence-based treatment update | 2014 | Systematic review | High |
| Hongfei et al.36 | Photodynamic therapy in the treatment of basal cell carcinoma: A systematic review and meta-analysis | 2015 | Systematic review and meta-analysis | Acceptable |
Data on Clinical Trials Not Included in the Systematic Reviews.
| Authors | Title | Type of Study | Year of Publication | Treatments Included | Interpretation/Conclusion |
|---|---|---|---|---|---|
| Beutner et al.19 | Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream | Randomized, double-blind | 1999 | Imiquimod | 100% histologic clearance with regimens of twice daily, once daily, and 3 times a week |
| Imiquimod 5% cream shows clinical efficacy in the treatment of BCC. | |||||
| Bath-Hextall et al.30 | Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicenter, non-inferiority, randomised controlled trial | Pragmatic multicenter noninferiority randomized controlled trial with parallel groups | 2014 | Surgical excision vs. imiquimod 5% cream | 3-y response rate of 84% (n=178) and 98% (n=185) in imiquimod and surgery groups, respectively |
| Imiquimod is a useful treatment option for small, low-risk superficial or nodular BCC. | |||||
| Avril et al.43 | Basal cell carcinoma of the face: Surgery or radiotherapy? Results of a randomized study | Randomized trial | 1997 | Surgery vs. RT | 4-y recurrence rate of 0.7% and 7.5% in surgery and RT groups respectively. Surgery should be chosen before RT in facial BCCs <4cm. |
| Sekulic et al.48 | Efficacy and safety of vismodegib in advanced basal-cell carcinoma | Nonrandomized international multicenter study with 2 cohorts | 2012 | Vismodegib | Overall response of 43% in patients with laBCC and 30% in those with mBCC |
| Basset-Seguin et al.49 | Vismodegib in patients with advanced basal cell carcinoma (Stevie): A pre-planned interim analysis of an international, open-label trial | Open multicenter trial | 2015 | Vismodegib | 302 (66.7%) of 453 patients with laBCC showed overall response (153 complete and 149 partial); 11 (37%) of 29 patients with mBCC showed overall response (2 complete and 9 partial) |
| Migden et al.51 | Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomised, double-blind phase 2 trial | Onoing multicenter double-blind randomized phase II clinical trial | 2015 | Sonidegib | Objective response in patients with laBCC was 43% for 200mg/d group and 38% for 800mg/d group; corresponding rates for patients with mBCC were 15% and 17%, respectively. |
| Rodon et al.52 | A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors | Phase I study | 2014 | Sonidegib | Maximum tolerated doses of 800mg/day and 250mg twice a day |
Abbreviations: BCC, basal cell carcinoma; laBCC, locally advanced BCC; mBCC, metastatic BCC; RT, radiation therapy.
To choose the most appropriate medical treatment for BCC, it is important to be familiar with the different predictors of local recurrence in order to categorize the risk for individual lesions (Table 3).
Classification of Basal Cell Carcinoma According to Risk of Recurrence.
| Low-risk |
| Locationa |
| H zone: <6mm |
| M zone <10mm |
| B zone: <20mm |
| Histologic subtype |
| Nodular |
| Superficial |
| Primary tumor |
| No perineural invasion |
| Well-defined borders |
| High-risk |
| Location |
| H zone: ≥6mm |
| M zone: ≥10mm |
| B zone: ≥20mm |
| Histologic subtype |
| Micronodular |
| Infiltrative |
| Morpheaform |
| Basosquamous |
| Mixed |
| Recurrent tumor |
| Perineural invasion |
| Poorly defined borders |
BCCs are classified as low-risk, medium-risk, or high-risk according to their location. High-risk tumors are located in the H-zone of the face (midface, lower perioribital region, temple, and preauricular and postauricular region). Medium-risk tumors (M zone) are located outside the H-zone, i.e., on the head and neck, and low-risk tumors (L zone) are located on the rest of the body (trunk and extremities)
Source: Adapted from Rueda et al.17
Histologically, BCC can be classified into 2 subtypes: an indolent-growth subtype (nodular and superficial BCCs) and an aggressive-growth subtype (morpheaform, infiltrative, micronodular, and basosquamous BCCs). Distinct histopathologic patterns can coexist in a single tumor, in which case the pattern is referred to as a mixed pattern.3,15–17
Locally advanced BCC is an important category. These tumors are characterized by long duration, a history of multiple recurrences, and considerable facial disfigurement resulting from tumor growth and previous surgery. The chance of surgical cure in such cases is low or nonexistent.
Medical Treatments for BCCNumerous medical treatment modalities have been described for BCC, including topical imiquimod, 5-fluorouracil (5-FU), photodynamic therapy (PDT), intralesional interferon (IFN), radiation therapy, and Hedgehog (Hh) pathway inhibitors (Table 4).
Nonsurgical Treatment Options for BCC.
| Treatment Modality | Subtype of BCC Treated | Short-term Results (<1 y) | Safety Profile | Level of Evidence | References |
|---|---|---|---|---|---|
| Topical imiquimod | Low-risk nodular and superficial | RR: 73%-92.7% | Good | Moderate/high | 20, 27, 28 |
| Topical 5-fluorouracil | Low-risk superficial | RR: 80.1%-93% | Good | Moderate/high | 32, 33 |
| Photodynamic therapy (methyl aminolevulinate) | Low-risk superficial | RR: 66.8%-86.5% | Good | Moderate | 20, 33 |
| Intralesional interferon | Nodular | RR: 86% | Good | Moderate | 41 |
| Radiation therapy | Nodular and superficial | RcR: 2% (2 y) 7.5% (4 y) | Good | Moderate | 43 |
| Vismodegib | Locally advanced and metastatic | RR: 27%-46% | Good | Moderate | 50 |
| Sonidegib | Locally advanced and metastatic | RR: 15%-47% | Good | Moderate | 52 |
Abbreviations: RcR, recurrence rate; RR, response rate.
Imiquimod 5% is a toll-like receptor agonist approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of primary superficial BCCs with a diameter of less than 2cm. It is believed to induce IFN-α, tumor necrosis factor-α, and other cytokines that activate T helper 1 cells, which, in turn, induce an antitumor immune response.18
The first study to evaluate imiquimod in the treatment of superficial BCC was published in 1999.19 It was a clinical trial that compared 3 treatment regimens: imiquimod twice a day, imiquimod once a day, and imiquimod 3 times a week. Histologically confirmed complete response was observed in the 3 groups on completion of treatment. The complete response rate was 60% in the group of patients who received 2 doses a week, and 50% in the group that received treatment once a week.
A systematic review of treatment success in primary superficial BCC published in 2012 by Roozeboom et al.20 reported an estimated complete response rate of 86.2% (95% CI, 82%-90%) for imiquimod after a follow-up period of 6 to 19 weeks. The 1-year tumor-free survival rate based on pooled estimates from 23 studies was 87.3% (95% CI, 84%-91%). The authors concluded that imiquimod was an effective treatment for superficial BCC with a maximum diameter of 2cm.
The British Association of Dermatologists guidelines for the management of BCC rate the use of topical imiquimod with a quality of evidence I and a strength of recommendation A.21 The use of histologic verification22–26 and follow-up periods of up to 5 years27–29 in the studies reviewed to draw up these guidelines adds strength to the effectiveness of imiquimod in the treatment of superficial BCC. The final study reports drawn up by Gollnick et al.27,28 showed 5-year histologic clearance rates of 85.4% and 86.9% for patients with superficial BCC treated with imiquimod 5 times and 7 times a week for a period of 6 weeks, respectively.
In 2014, the results of a multicenter clinical trial involving 501 patients with superficial or nodular BCC (not including BCC of the nose, forehead, eyelids, or ears) randomly assigned to daily treatment with imiquimod for 6 weeks (n=254) or surgical excision (n=247) were published.30 After 3 years of clinical follow-up, 84% of patients in the imiquimod group and 98% of those in the surgery group had no signs of recurrence.
In brief, imiquimod can be considered an effective treatment alternative for patients with small, primary superficial BCCs at low-risk anatomic sites and for patients in whom surgery is contraindicated or who choose not to undergo surgery.31
Topical 5-FUAlthough topical 5-FU was the first topical treatment to receive approval from the FDA for the treatment of superficial BCC, the drug has not been widely studied in this cancer. 5-FU interferes with DNA synthesis by blocking the methylation of deoxyuridylic acid, thereby inhibiting thymidylate synthetase and consequently cell proliferation. The study that led to the approval of 5-FU by the FDA reported a success rate of 93% for the use of imiquimod in 113 superficial BCC lesions in 54 patients.32
A Dutch multicenter study published in 2013 evaluated the efficacy of 3 treatments for superficial BCC with a follow-up period of 12 months: topical 5-FU (twice daily for 4 weeks), imiquimod (once daily 5 times a week for 6 weeks), and PDT with methyl aminolevulinate (2 sessions separated by 1 week).33 The estimated overall success rates, assessed by the proportion of patients free of tumor recurrence at 3 months and 12 months, were 80.1% for 5-FU, 83.4% for imiquimod, and 72.1% for PDT.
In brief, topical 5-FU is an effective topical treatment for patients with primary superficial BCC at low- or medium-risk sites or for patients who are not candidates for surgery or who have high cosmetic expectations.32
Photodynamic TherapyPDT is another medical treatment used in BCC. It uses visible light to activate a photosensitizing agent, which can be aminolevulinic acid or methyl aminolevulinate (MAL), to produce reactive oxygen species that destroy tumor cells through an intracellular product known as protoporphyrin IX.34 Success rates of around 75% have been reported for PDT with MAL in studies that have compared efficacy and cosmetic outcomes over 1 year with that of conventional surgical excision for superficial BCCs.31,35
A systematic review from 2012 that analyzed 1583 patients with superficial BCC reported that the efficacy of PDT may depend to a large extent on the number of cycles used. The pooled estimate of complete response, for instance, increased from 76.6% to 79% (95% CI, 62%-90%) when repeated cycles were used. Tumor-free survival also increased from 76.2% to 84%.20
A comparison of the efficacy of PDT in terms of cosmetic outcome and safety with that of other treatments for primary BCC showed that compared with surgery, PDT was associated with a lower complete clearance rate (risk ratio [RR], 0.93; 95% CI, 0.89-0.98) and a higher probability of recurrence at 1 year (RR, 12.42; 95% CI, 2.34-66.02) and 5 years (RR, 6.79; 95% CI, 2.43-18.96).36
The study also showed that PDT has a higher complete clearance rate than placebo. No significant differences were observed with pharmacologic treatment (imiquimod and topical 5-FU) for complete clearance or 1-year recurrence, although PDT performed worse in the second case. One-year recurrence rates for PDT ranged between 6% and 44%, although lower rates were seen with multiple treatments (≥ 2 cycles on days 1 and 8). Clearance rates of between 76% and 79% were reported.36
PDT can be considered a treatment option for low-risk BCC (mainly small tumors, superficial tumors, tumors in low-risk locations) or for patients who are willing to trade a higher risk of recurrence for a better cosmetic outcome, or in whom surgery is contraindicated for medical reasons.36
Intralesional IFNIFN is one of several intralesional chemotherapy agents currently used in the management of BCC. It stimulates macrophages, natural killer cells, and lymphocyte-mediated cytotoxicity to induce an antitumor immune response.37
The first clinical trial with this drug included 172 patients with histologically confirmed noduloulcerative or superficial BCC who were treated with either intralesional IFN-α2b or placebo.38 The patients in the IFN group received 1.5 million international units (IU) 3 times a week for 3 weeks, resulting in a cumulative dose of 13.5 million IU. The cure rate was 86%.
A systematic review designed to analyze the evidence currently available for the treatment of BCC published in 2014 reported a histologic clearance rate of 85% for INF-α2b and excellent cosmetic outcomes in 83% of cases. The most common adverse effects reported by all patients were flu-like symptoms consisting of headache, myalgia, and nausea.12,37
Intralesional IFN can offer advantages over traditional techniques in the treatment of BCC, particularly when surgery is not an option or when the tumor is in a cosmetically delicate area.39
Radiation TherapyRadiation therapy is a classic technique that was used as a first-line treatment for BCC for several years. It is now, however, used frequently for a number of reasons.7,31 It is currently indicated as a second-line treatment for high-risk tumors or for patients in whom surgery is contraindicated.40,41
A retrospective analysis of 712 BCCs (631 nodular and 81 superficial tumors) treated with 5 sessions of superficial x-ray therapy at a total dose of 35Gy showed recurrence rates of 2% at 2 years and 4.2% at 5 years.42 In another study, a randomized clinical trial, comparison of surgical excision of 174 BCCs (primary facial tumors <4cm in diameter with a nonaggressive histologic subtype) and radiation therapy of 173 BCCs with similar characteristics showed a 4-year recurrence rate of 0.7% in the surgery group and 7.5% in the radiation therapy group.43
The results for the use of radiation therapy in BCC vary considerably in the literature, possibly because of differences in treatment regimens and the lack of an optimal regimen.44
Radiation therapy, however, has several disadvantages, including absence of histologic verification, long treatment times requiring multiple visits, cosmetic outcomes that tend to worsen with time (cutaneous atrophy and telangiectasias), and a risk of new radiation-induced BCCs.31
In short, radiation therapy is a valuable treatment option for patients with BCCs with a high risk of recurrence who are not candidates for surgery, patients aged over 60 years (due to the likelihood of long-term complications), and patients with primary or recurrent tumors. It can also be used as adjuvant therapy after surgery or in cases with perineural invasion or involvement of margins.
Hh Pathway InhibitorsVismodegibIn 2012, the FDA approved a new class of drug for the treatment of locally advanced and metastatic BCC: vismodegib. Molecular and genetic studies have shown genetic alterations of the Hh signaling pathway in almost all BCCs. These alterations result in aberrant activation of the pathway and as a result the uncontrolled proliferation of keratinocytes. More commonly, they cause loss of function of the patched 1 homolog gene (PTCH1), which generally exerts an inhibitory effect on the signal transducer Smoothened (Smo), which in turn triggers the effector cascade invoked by the Gli protein complex (a family of glioma-associated oncogene transcription factors). Over 90% of sporadic BCCs and the vast majority of BCCs that occur in Gorlin syndrome have PTCH1 mutations.45 Vismodegib is a small molecule that specifically binds to and inactivates Smo, preventing the activation of the pathway through suppression of proliferation and tumor growth.46-48
Vismodegib is indicated for the treatment of BCC in adults with locally advanced or metastatic tumors who are not candidates for surgery and in whom radiation therapy has failed.
The landmark phase II vismodegib study (Erivance) of 104 patients with locally advanced or metastatic BCC reported an overall response rate of 43% for patients with locally advanced BCC and 30% for those with metastatic BCC.48 After a follow-up period of 30 months, the overall objective response rate, assessed by site investigators, was 60% in the group of patients with locally advanced BCC and 48.5% in the group with metastatic BCC. These percentages were comparable to those of the primary analysis. Duration of objective response was 26.2 months for the patients with locally advanced BCC and 14.8 months for those with metastatic BCC. The respective disease-free survival times were 12.9 and 9.3 months.48
The multicenter clinical trial with the largest number of patients treated to date with vismodegib (Stevie) was designed to assess the safety of the drug.49 Preliminary results reported for the first 499 patients who completed a year of follow-up (n=468 with locally advanced BCC and n=31 with metastatic BCC) showed a median duration of exposure to the drug of 36.4 weeks. Based on the evaluation by the site investigators, 302 (66.7%) of 453 patients with locally advanced BCC responded to treatment: 153 (34%) showed complete response, 149 (33%) showed partial response, and 118 (26%) showed stable disease. Of the 29 patients with metastatic BCC, 11 (37.9%) responded; the response was partial in 9 cases (31%) and complete in 2 (7%). Ten patients (34%) had stable disease. Adverse effects were reported in approximately 98% of patients (490/499) (Table 5). These events were, by order of frequency, muscle spasms (64%), alopecia (62%), dysgeusia (54%), weight loss (33%), asthenia (28%), decreased appetite (25%), ageusia (22%), diarrhea (17%), nausea (16%), and fatigue (16%).
Adverse Events Reported for Smoothened Inhibitors Vismodegib and Sonidegib.
| Adverse Event | Vismodegib (150mg) | Sonidegib (200mg) | Sonidegib (800mg) |
|---|---|---|---|
| n=499 | n=79 | n=150 | |
| Muscle spasms | 64% | 49% | 67% |
| Alopecia | 62% | 43% | 55% |
| Dysgeusia | 54% | 38% | 59% |
| Weight loss | 33% | 27% | 38% |
| Asthenia | 28% | 8% | 5% |
| Decreased appetite | 25% | 29% | 36% |
| Ageusia/hypogeusia | 22% | 0% | 5% |
| Diarrhea | 17% | 24% | 22% |
| Fatigue | 16% | 29% | 36% |
| Nausea | 16% | 33% | 45% |
In total, 22% of patients experienced severe adverse events; the most common were pneumonia, general deterioration in health, squamous cell carcinoma, and dehydration. Six percent of the patients died. Twenty-one of the deaths were attributed to adverse events, 5 to disease progression, and 5 to other causes.49 A recent analysis of data from the Stevie study showed that temporary suspension of vismodegib for 2, 3, or 4 weeks (up to 8 weeks according to protocol) does not interfere with the efficacy of treatment in terms of disease-free survival and response rates.50
Vismodegib is thus another treatment alternative for advanced or metastatic BCC in patients who relapse following radiation therapy and/or in whom surgery is inappropriate or contraindicated.49
SonidegibThe FDA and EMA recently approved another Hh pathway inhibitor, sonidegib. In the multicenter randomized double-blind phase II Basal Cell Carcinoma Outcomes in LDE225 trial that led to the approval of sonidegib for BCC,51,52 230 patients were assigned to one of 2 groups in which they received an oral dose of 200mg or 800mg daily. Of the 230 patients, 193 had locally advanced BCC and 37 had metastatic BCC.51
The objective response rates in the group of patients with locally advanced disease were 43% for the 200-mg dose and 38% for the 800-mg dose. The respective rates in the group of patients with metastatic disease were 15% and 17%. Median duration of treatment was longer in the lower-dose group (8.9 vs 6.5 months) and this longer treatment time was attributed to the lower incidence of adverse effects. The most common adverse events were similar to those seen with vismodegib (Table 5).51
Based on the results of this trial, sonidegib was approved at a dose of 200 mg once daily for the treatment of adult patients with locally advanced BCC who experience recurrence following surgery or radiation therapy or who are not candidates for these treatments.53
ConclusionsNonsurgical treatments for BCC are effective, particularly in the case of low-risk subtypes. They can be considered a treatment alternative for patients who do not want to undergo surgery or in whom surgery is contraindicated. Hh pathway inhibitors have opened up new treatment possibilities for the rarer cases of advanced BCC, showing promising efficacy and a good safety profile, although more studies are needed to define optimal treatment schedules.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe first author reports having received speakers’ fees for presentations on vismodegib from Productos Roche-Colombia.
The co-authors declare that they have no conflicts of interest.
We thank the research division at the Fundación Universitaria de Ciencias de la Salud, and in particular Diana Buitrago for her dedication and help in the production of this manuscript.
Please cite this article as: Ariza S, Espinosa S, Naranjo M. Nonsurgical Therapies for Basal Cell Carcinoma: A Review. Actas Dermosifiliogr. 2017;108:809–817.