Human papillomavirus infection is very common. In this article, we review the latest developments in the treatment of lesions caused by this virus, with a particular focus on anogenital warts.
Sinecatechins and new imiquimod formulations are among the most significant new developments. Others include photodynamic therapy and intralesional immunotherapy, but there is insufficient evidence to recommend their routine use. Finally, while therapeutic vaccines and inhibitory molecules appear to hold great promise, they are still in the early phases of investigation.
More studies are needed, and these should have similar designs, larger samples, and sufficiently long follow-up periods to enable the direct comparison of the short-term and long-term effectiveness of different treatment options.
La infección por el virus del papiloma humano es una afección muy prevalente. Se revisan los aspectos más novedosos del tratamiento de las lesiones producidas por este virus, especialmente las verrugas anogenitales.
Las sinecatequinas y las nuevas formulaciones de imiquimod destacan como novedades, mientras que la terapia fotodinámica y la inmunoterapia intralesional carecen de evidencia científica suficiente para recomendar su uso rutinario. Las vacunas terapéuticas y las moléculas inhibidoras parecen poseer un gran potencial, aun cuando se encuentran en fases iniciales de investigación.
Sería deseable disponer de estudios más homogéneos, con muestras más grandes y con seguimientos suficientemente prolongados que permitiesen comparar directamente la efectividad entre las diferentes modalidades terapéuticas a corto y largo plazo.
Human papillomavirus (HPV) causes common and anogenital warts and infection is associated with precancerous dysplasia and cancer, specifically with penile, vaginal, vulval, anogenital, and oropharyngeal carcinomas.1 More than 180 HPV genotypes have been identified.2 The carcinogenic potential of HPV 16 and 18 is well known: these genotypes are found in up to 70% of carcinomas of the uterine cervix. HPV 6 and 11 are associated with low oncogenic risk and are responsible for viral anogenital warts. Other genotypes are associated with viral warts at other anatomic sites such as palms and soles, dorsum of the hands, face, hairless skin, mucosas, etc.
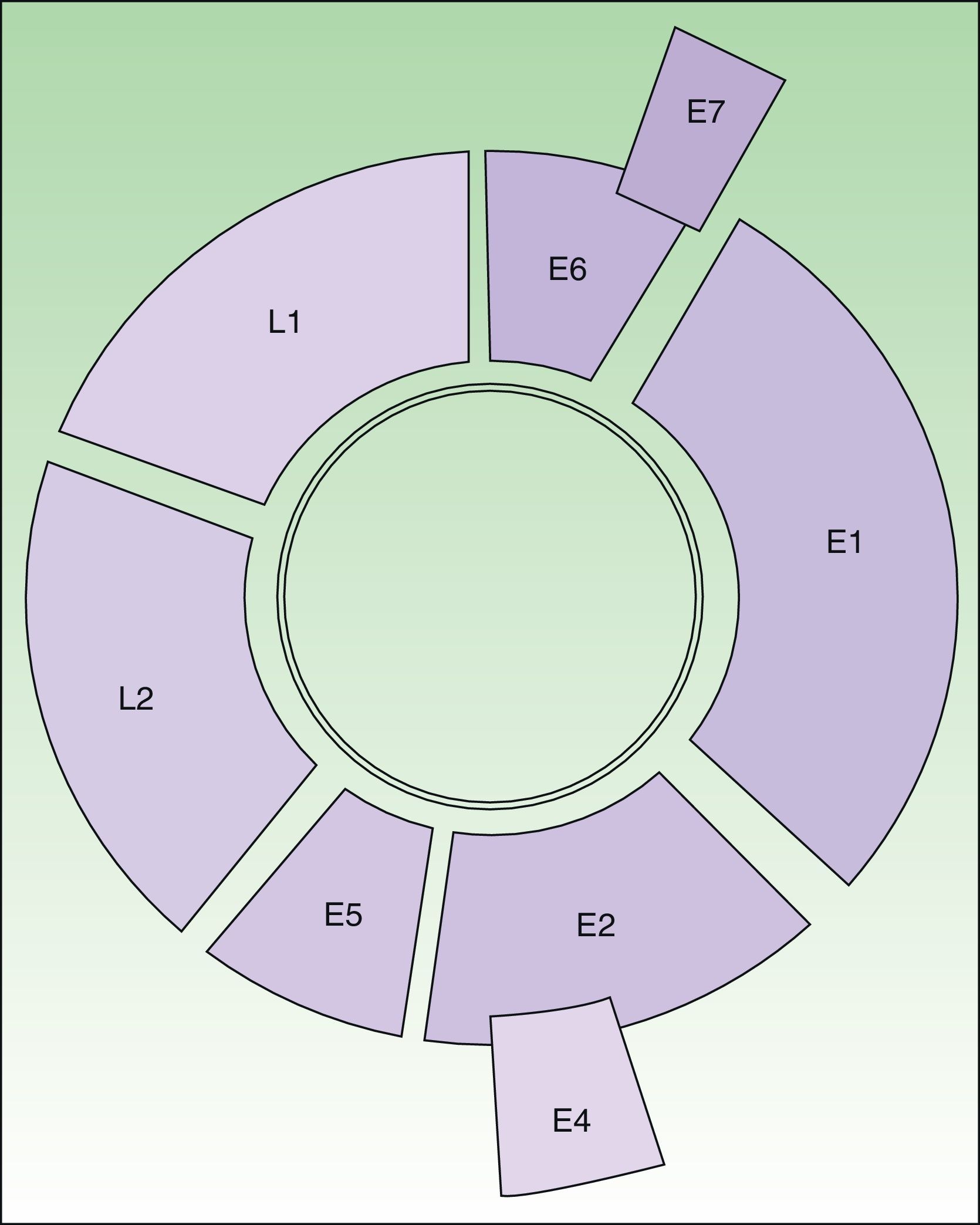
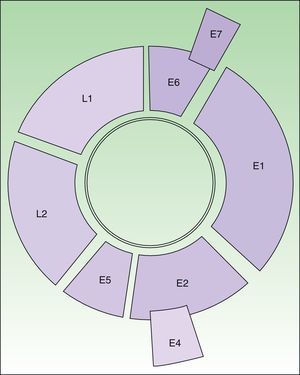
HPV is a double-stranded-DNA virus that encodes 8 genes1,2 (Fig. 1). L1 and L2 encode the structural proteins that form the capsid. E6 and E7 interfere with normal cell activity. E6 encodes an oncoprotein that binds to p53, thereby inhibiting apoptosis. The protein encoded by E7 binds to pRB, thereby arresting the cell cycle. The combined expression of these proteins promotes genomic and chromosomal instability. The last step in carcinogenesis is the integration of viral DNA in the cell genome.
Diagram of the HPV 16 genome (8000 base pairs). Circular, double-stranded DNA molecule. E6 and E7 are oncogenes. E1 participates in viral DNA replication (helicase activity). E2 encodes an auxiliary protein that affects replication and is also involved in transcriptional regulation. E4 disrupts cytokeratins, facilitating their release. E5 stimulates cell growth by interacting with receptors. Capsid proteins are encoded by L1 (major) and L2 (minor); expression is late in both cases. The regulatory region is located between L1 and E6.
Various treatments are available for managing viral warts but none has clearly proven superior. Patient and physician should reach agreement regarding the need for—and choice of—treatment, taking into account the morphology and extent of the lesions. It is essential for the physician to examine the patient at least every 4 weeks to assess changes in the lesions and modify the treatment in the event of inadequate response.3 Treatments can be classified by whether they are applied by the physician in the clinic or by the patient at home. They can also be classified by objective: some treatments are designed to eliminate the virus by means of direct action or immunomodulation, whereas others are intended to simply destroy the infected cells. The various available therapies include salicylic acid, lactic acid, retinoic acid, cantharidin, bleomycin, 5-fluorouracil, interferon alfa, podophyllotoxin (0.5% solution, 0.15% cream), imiquimod (5% cream), cidofovir (1% cream or intralesional application), trichloroacetic acid (80%-90% solution), cryotherapy, electrosurgery, surgical excision, curettage, and laser treatment (carbon dioxide, yttrium-aluminum-garnet). In the sections that follow, we review the latest developments, especially in the treatment of external anogenital warts.
New Developments in TreatmentImiquimodNew formulations of imiquimod cream at concentrations of 2.5% and 3.75% are now available on the market. The United States and Canada recently approved imiquimod 3.75% cream for use in the treatment of external anogenital warts (Zyclara 3.75%). This cream is applied once daily until the lesions resolve or for a maximum of 8 weeks. In phase III trials, complete clearance was achieved in 29% of patients (vs 10% of patients who used a placebo cream) and the best results were seen in women (complete clearance in up to 37% of cases). Imiquimod 3.75% and imiquimod 2.5% have been compared in only 1 placebo-controlled study,4 in which 534 women were enrolled. Eight weeks after the start of treatment, complete remission was achieved in 36.6% of patients who received imiquimod 3.75% and in 14.2% of patients in the placebo group (P<0.001).4 On average, the number of lesions decreased by 63.5% in the imiquimod 3.75% group and by only 10.7% in the placebo group. Imiquimod 3.75% was more effective than the 2.5% formulation in both comparisons (complete remission and percentage of lesions cleared)—though not to a statistically significant degree—and the higher concentation produced no increase in undesirable effects. It is difficult to directly compare the effectiveness of imiquimod 5% cream with that of the new formulation because no studies have directly compared the two concentrations and because there are major design differences between the studies that have investigated the formulations separately. However, the results of phase III trials show that the new concentration is much better tolerated and less likely to cause local skin reactions than the 5% formulation.4,5 Daily application of imiquimod 3.75% cream can be considered a new treatment option for genital warts, especially in women, in whom this concentration appears to be more effective.4,5 The main advantages of this new formulation are that it simplifies and shortens the treatment regimen (application each night for a maximum of 8 weeks) and is better tolerated, encouraging better adherence to therapy.5 The new imiquimod 3.5% formulation is not yet commercially available in Spain and we do not know how much it will cost here.
Sinecatechins (Polyphenon E)Polyphenon E is a standardized extract of green tea leaves (Camellia sinensis). Epigallocatechin gallate, the most abundant catechin in green tea, plays a role in multiple cell signaling pathways, activating the caspase pathway, regulating Bcl-2 expression, and inhibiting telomerase.1 By means of these mechanisms, epigallocatechin gallate inhibits the cell cycle, activates apoptosis, inhibits HPV transcription, and activates the cellular immune response.6 The combination of these various actions eliminates clinically affected cells as well as cells with subclinical infection.
In the United States, an ointment containing sinecatechins at a concentration of 15% (Veregen 15%) has been approved for the treatment of external anogenital warts. The dosage is 3 applications daily for up to 16 weeks. Randomized controlled trials in patients of both sexes have shown overall lesion clearance rates of between 54% and 65% compared to an average clearance rate of 35% in placebo groups.7–10 The wart clearance rate of the placebo groups in these clinical trials is noteworthy, as it is higher than the known clearance rates achieved with other treatments against condylomata. This could be due to the fact that, according to the product description, the potentially irritating excipients isopropyl myristate and propylene glycol are used in the formulation of both the ointment and the vehicle. Recurrence rates were between 6% and 12% after 12 weeks of follow-up. A 10% ointment was also used in these studies, with similar results. This concentration has recently become commercially available in Spain (Veregen 100mg/g ointment marketed by Bial; a 15 g tube costs €60).
The effect of this substance is not evident clinically until approximately the third week of treatment and becomes more apparent in the fourth to sixth weeks.7 The most common undesirable effects (80%) are local ones, particularly erythema and pruritus, that begin to appear in the second or third week of treatment.7–10 Although a large percentage of patients have adverse reactions, they are well tolerated. Inflammation, indicative of the drug's activity, arises from a local immune response mediated by proinflammatory cytokines. The incidence of local skin reactions has been reported to be higher in responders than nonresponders.8 In short, given that few patients abandon treatment because of undesirable effects, this formulation seems to be an effective, safe, and well-tolerated therapeutic option. However, the length of the treatment regimen—16 weeks, with 3 applications daily—can have a negative impact on adherence. Because most randomized controlled trials have excluded transplant recipients and patients with human immunodeficiency virus (HIV) infection, the results are limited to immunocompetent individuals. The effectiveness of this drug in the treatment of internal warts is not known.
Studies comparing sinecatechins to imiquimod over long follow-up periods are needed so that the rates of lesion clearance and, in particular, recurrence can be compared.
Because of their well-established antiviral and antineoplastic activities, topical sinecatechins may also potentially be useful in the treatment of nonmelanoma skin cancer and infections caused by the herpes simplex or varicella zoster viruses.6
Photodynamic TherapyPhotodynamic therapy (PDT) is based on the immune response to tissue destruction caused by a phototoxic reaction. 5-aminolevulinic acid has been reported to accumulate in HPV-infected cells in greater quantities than in adjacent normal skin.11 PDT has been proposed for treating refractory lesions and lesions that recur despite the correct administration of another treatment. PDT can also be used to treat cervical or vulvar intraepithelial neoplasia.12
A trial of PDT with white light and 5-aminolevulinic acid in combination with keratolytic agents in patients with warts on the palms and soles that had been resistant to other treatments13 found that the wart clearance rate at 18 weeks was 56% after 6 sessions of PDT. There is less evidence to support the use of methyl aminolevulinate (MAL) PDT. The combination of MAL PDT and carbon dioxide laser therapy has been proposed for periungual warts.14 More studies have been carried out on the use of PDT in the treatment of external genital warts. The findings suggest that 1 or 2 sessions of PDT with 5-aminolevulinic acid can be as effective as excision or laser treatment, with lower rates of recurrence at 12 weeks.15,16 It appears that the best results are achieved with 16% to 20% gel formulations of 5-aminolevulinic acid and a red light dose of 100-150J/cm2.17 The adverse effects, all local, include pain, a burning sensation, and erythema.17 The therapeutic effect of PDT in condylomata is attributable to the rapid activation of dendritic cells and CD4+ T cells in the lesional skin.18 The main advantages of PDT are a high degree of effectiveness and safety, a short recovery period, and good cosmetic results.12 The main problems associated with PDT are pain during exposure, the need for a red light source, the difficulty of applying occlusive dressings to lesions in the anogenital area, and the need to occasionally perform curettage or inject the photosensitizer into larger or thicker lesions in order to enhance penetration and increase its effectiveness, and the cost of topical MAL (which costs €280 for 2g).
Intralesional ImmunotherapyReports of single cases and small series of patients receiving intralesional immunotherapy for the treatment of recalcitrant warts—especially plantar and periungual lesions—have been published in recent years.19–21 Both adults and children have been treated with this method, which uses the immune system's capacity to mount a type 1 helper T cell (TH1)-mediated delayed-type hypersensitivity response to various antigens, including HPV. This response is associated with the production of cytokines that activate CD8 lymphocytes and natural killer cells, which are necessary for the eradication of HPV infection.22 Unlike conventional treatments, intralesional immunotherapy can eradicate not only the treated wart but also distant lesions22; the antigen is therefore usually only injected into the largest wart. Various antigens have been used, but the best known ones are the Candida antigen and the mumps virus antigen. More recently, single case reports and small case series have described the results of infiltration of tuberculin purified protein derivative (PPD)19; mumps, measles, and rubella (MMR) vaccine20,21; and Mycobacterium w vaccine (for anogenital warts).23 Infiltrations of between 0.1 mL and 0.3mL are usually administered at intervals of 1 to 3 weeks for a maximum of 3 to 5 sessions. Intralesional immunotherapy achieves highly satisfactory results at a reasonable cost. Candida antigen is usually supplied in kits used by allergists to perform intradermal tests. Because they are designed to be used in multiple patients, it can be expensive to use these kits to treat a single patient or only a few. Other substances used in intralesional immunotherapy (vaccines, PPD) cost approximately €9 to €15 per vial, and each vial allows the application of more than 1 dose. The procedure can be approached in 2 ways. One way is to inject the antigen directly into the warts. In an alternative approach, the antigen is injected intradermally in the patient's forearm and the local hypersensitivity reaction (erythema and induration of ≥5mm in diameter) is assessed 48 to 72hours later. If positive, warts are then injected with a volume proportional to the size of the reaction.22 These treatments are reserved for recalcitrant lesions because they are not approved for this indication and their use is not supported by well-designed clinical trials. Furthermore, it is difficult to compare the use of different antigens because the treatment and follow-up regimens are dissimilar. The most common side effects, all mild, are an inflammation at the injection site and flu-like symptoms.22
Prophylactic Vaccines Used TherapeuticallyTwo prophylactic HPV vaccines are available: one is a quadrivalent vaccine against HPV types 6, 11, 16, and 18 and the other is a bivalent one against types 16 and 18. Today's vaccines are composed of purified HPV L1 structural protein produced by recombinant technology and contain no HPV genetic material. These virus-like particles induce a humoral immune response to the virus itself. High levels of both systemic and local antibodies are found in the genital mucosa.1 The humoral response, in turn, appears to stimulate a cellular immune response. In Spain, the cost of 3 doses of quadrivalent HPV vaccine is approximately €450.
Since these prophylactic vaccines were approved in 2006, there have been cases of remission of recalcitrant warts (on the hands and soles) in patients treated as usual (not by intralesional injection) with the quadrivalent vaccine.24–26 The exact mechanism for this clinical response is obscure and it is unclear whether the spontaneous regressions occurred coincidentally. There have been no trials of HPV vaccine used in the treatment of warts. It is possible that the vaccine provides cross-protection with antibodies against other HPV genotypes present in warts. As in therapeutic vaccines, some local cytokines could be involved through the induction of interferon-producing cells or cytotoxic CD8 lymphocytes. It is also possible that the non-HPV components of the prophylactic vaccine play a role.26
Therapeutic VaccinesVaccines that target the E6 and E7 oncoproteins of oncogenic HPV genotypes have also been explored as a treatment option. These vaccines stimulate a cellular immune response in which T cells—both TH1 and CD8+—act against these target proteins.27
In the future, therapeutic vaccines could be used to treat HPV-related precancerous lesions, which consistently express these proteins. Various types of vaccines have been developed. Peptide-based vaccines—which are safe, stable, and easy to produce—are promising.28
More research on these vaccines is needed. Clinical evidence is lacking because there have been no well-designed clinical trials with a sufficient number of patients.
Molecular Inhibitors of Human Papillomavirus ProteinsCurrently, there are no specific molecular inhibitors that directly target HPV. These drugs are needed for several reasons. First of all, because these antiviral drugs act on viruses directly, they affect imperceptible lesions as well as clinically apparent ones. Such drugs are also needed to treat multifocal disease such as vulvar or anal intraepithelial neoplasia, which cannot always be treated with ablation. Finally, immunocompromised patients would also benefit from the development of molecular inhibitors because HPV elimination and immunotherapy require a normally functioning immune system.
The fact that E1 helicase is the only enzyme that encodes HPV has been an obstacle to the development of inhibitors.2,29 The various HPV proteins and their interactions are currently being investigated with the aim of creating new direct antiviral drugs that act by inhibiting the E1 and E2 proteins or their interaction, the binding of the E2 with cellular proteins (small-molecule inhibitor JQ1),2 or E6 and E7. Antivirals acting on E6 and E7—which play an essential role in HPV oncogenesis—have been designed to prevent formation of the complex responsible for p53 degradation.1,29 Research also targets inhibitors that can block HPV from entering cells after exposure. One such inhibitor is carrageenan, a seaweed extract that binds to the L1 capsid to prevent HPV from attaching to basement membrane proteoglycans in epithelial tissues.1
These strategies are still in the very early stages of investigation, and since the advent of vaccines, some research into direct antiviral agents has been suspended. It will surely be years before any inhibitors come to form part of the therapeutic arsenal against HPV.
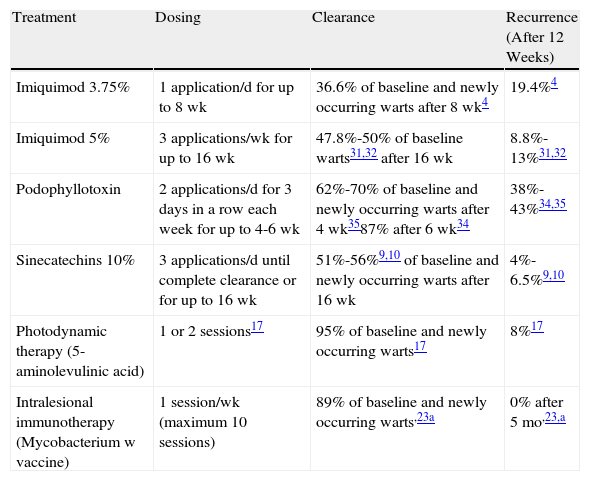
ConclusionAnogenital warts rarely cause major symptoms or complications; they are usually treated for cosmetic or psychological reasons and we should remember that they can resolve spontaneously (in up to 30% of cases).6 Sinecatechins are undoubtedly the most valuable recent addition to the therapeutic arsenal against HPV. The available evidence indicates that sinecatechins are efficacious and have a good safety profile. When comparing sinecatechins and imiquimod, differences in study design must be taken into consideration. The effect of sinecatechins on both baseline warts and lesions that appeared during treatment or follow-up was evaluated 16 weeks after the start of treatment. In contrast, the effect of imiquimod 5% cream was evaluated based on only the warts that were present at the start of the study.30–32 The efficacy of sinecatechins may therefore be underestimated. A cost-effectiveness analysis of these 2 drugs concluded that sinecatechins were the more favorable alternative.33Table 1 summarizes and compares the new and conventional treatments.
Summary of New Treatment Options for Anogenital Warts and Comparison with the Main Conventional Topical Treatments.
| Treatment | Dosing | Clearance | Recurrence (After 12 Weeks) |
| Imiquimod 3.75% | 1 application/d for up to 8 wk | 36.6% of baseline and newly occurring warts after 8 wk4 | 19.4%4 |
| Imiquimod 5% | 3 applications/wk for up to 16 wk | 47.8%-50% of baseline warts31,32 after 16 wk | 8.8%-13%31,32 |
| Podophyllotoxin | 2 applications/d for 3 days in a row each week for up to 4-6 wk | 62%-70% of baseline and newly occurring warts after 4 wk3587% after 6 wk34 | 38%-43%34,35 |
| Sinecatechins 10% | 3 applications/d until complete clearance or for up to 16 wk | 51%-56%9,10 of baseline and newly occurring warts after 16 wk | 4%-6.5%9,10 |
| Photodynamic therapy (5-aminolevulinic acid) | 1 or 2 sessions17 | 95% of baseline and newly occurring warts17 | 8%17 |
| Intralesional immunotherapy (Mycobacterium w vaccine) | 1 session/wk (maximum 10 sessions) | 89% of baseline and newly occurring warts,23a | 0% after 5 mo,23,a |
The lack of large well-designed studies of PDT and intralesional immunotherapy restricts the use of these treatments to refractory lesions. Few studies have evaluated the effect of these new approaches on mucosal warts in patients who are immunocompromised because of HIV infection or an organ transplant.
In conclusion, we highlight the general lack of evidence on the treatment of this very common infection. There is a need for trials with comparable designs and sufficiently long follow-up periods that directly compare the effectiveness of the various available treatments. Finally, the development of therapeutic vaccines and small-molecule inhibitors of HPV is a vast, exciting field of research that is sure to translate into clinical practice in the near future.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their hospitals concerning the publication of patient data and that all patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Muñoz-Santos C, Pigem R, Alsina M. Nuevos tratamientos en la infección por virus del papiloma humano. Actas Dermosifiliogr. 2013;104:883–889.