Vitiligo is an acquired skin disorder characterised by progressive depigmentation of the skin due to selective loss of melanocytes. Its aetiopathogenesis is complex and multifactorial, involving an interaction between genetic, immunological and environmental factors. Recent evidence leans towards an integrated model in which autoimmunity, mediated mainly by cytotoxic T lymphocytes and inflammatory cytokines, plays a central role. In addition, oxidative stress contributes significantly to melanocyte dysfunction and apoptosis. Genetic studies have identified numerous loci associated with vitiligo, most notably the involvement of genes related to adaptive and innate immunity as well as cellular metabolism. Environmental factors such as trauma, chemical exposure and psychosocial stress may also act as triggers in predisposed individuals. This review synthesises the most recent advances in the aetiopathogenesis of vitiligo, providing a comprehensive overview of the mechanisms involved and opening up new possibilities for the development of therapeutic approaches based on this knowledge.
El vitíligo es un trastorno cutáneo adquirido caracterizado por la despigmentación progresiva de la piel debido a la pérdida selectiva de melanocitos. Su etiopatogenia es compleja y multifactorial, involucrando una interacción entre factores genéticos, inmunológicos y ambientales. Las evidencias recientes se inclinan hacia un modelo integrado en el que la autoinmunidad, mediada principalmente por linfocitos T citotóxicos y citoquinas inflamatorias, desempeña un papel central. Además, el estrés oxidativo contribuye significativamente a la disfunción y apoptosis de los melanocitos. Estudios genéticos han identificado numerosos loci asociados con el vitíligo, destacando la participación de genes relacionados con la inmunidad adaptativa e innata, así como con el metabolismo celular. Asimismo, factores ambientales como el trauma, la exposición a sustancias químicas y el estrés psicosocial pueden actuar como desencadenantes en individuos predispuestos. Esta revisión sintetiza los avances más recientes en la etiopatogenia del vitíligo, ofreciendo una visión completa de los mecanismos implicados, y abriendo nuevas posibilidades para el desarrollo de enfoques terapéuticos basados en este conocimiento.
Vitiligo is a depigmenting skin disorder characterized by the selective loss of melanocytes. Its prevalence ranges from 0.5% to 2%, according to an extensive review of prevalence data from over 50 studies worldwide, with no predilection for gender or race.1
For years, the etiopathogenesis of vitiligo has been a subject of study, and although various theories have been proposed, many questions still remain about its precise causes and underlying mechanisms. In this article, we will explore the most recent research that sheds light on the complex interaction of genetic, autoimmune, neurogenic, and environmental factors contributing to the development and progression of vitiligo. Through a comprehensive review of current literature, we aim to offer an updated view of advances in understanding this enigmatic disease, as well as new therapeutic perspectives emerging from this knowledge.
Immunological mechanisms involved in the etiopathogenesis of vitiligoInnate immunityThe innate immune system is considered the crucial link connecting oxidative stress with the adaptive immune response in the development of vitiligo. In lesional and perilesional skin of patients, dendritic cells, macrophages, activated NK cells, and IFN-γ-producing cells are found. Melanocytes, through exosomes, communicate stress to the innate immune system, especially to dendritic cells that present antigens to T lymphocytes. In vitiligo patients, an increase in pro-inflammatory cytokines typical of innate immunity, IL IL-1α, IL-1β, IL-6, IL-8, IL-12, IL-15, and TNF-α, is observed in both serum and skin.2,3
Damage-associated molecular patternsThe Koebner phenomenon is considered an initial trigger for vitiligo. Attempts have been made to identify factors released during stress response and melanocyte damage in this disease. Damage-associated molecular patterns (DAMPs) are of particular interest, as they can trigger the inflammatory response observed in the active stage of vitiligo, with a series of DAMP-associated proteins existing in vitiligo.4
The DAMP with the most evidence of association with vitiligo is heat shock protein 70 (HSP70), which belongs to the family of intracellular chaperones whose function is to prevent incorrect protein folding. The overexpression of HSP70 in active vitiligo lesions indicates a possible role of this protein in the immune response and inflammation associated with the disease. Furthermore, HSP70 could be considered a potential marker to evaluate disease activity.3,5,6
On the other hand, MxA protein (human mixovirus resistance protein 1), inducible by IFN-α, shows strong expression in active perilesional vitiligo skin, suggesting a contributing effect of IFN-α in disease progression.7 Similarly, S100B is another DAMP released by damaged melanocytes, whose levels are increased in active vitiligo and can stimulate inflammatory responses.8 Also, high-mobility group box 1 (HMGB1) proteins can induce the production of chemokine ligands, such as CXCL1 or IL-8 by keratinocytes, acting in the recruitment of immune cells.9
Calreticulin (CRT) is another of the most studied molecules in vitiligo, as it induces melanocyte apoptosis and the release of membrane degradation products important for immunogenicity.10
Melanocyte adhesion deficiencySeveral research groups have demonstrated that melanocytes in vitiligo exhibit reduced adhesive properties. Thus, altered levels of E-cadherin expression have been observed in melanocytes from vitiligo-affected skin before depigmentation develops. Deficient E-cadherin expression leads to the loss of epidermal melanocyte adhesion during situations of oxidative or mechanical stress.11
Furthermore, a relevant role of integrins and the Melanoma Inhibitory Activity (MIA) protein has been identified. Integrins are involved in the interaction of melanocytes with the extracellular matrix, and their dysfunction can contribute to melanocyte loss. On the other hand, MIA protein, originally studied in melanoma, has shown a pro-apoptotic effect on melanocytes, favoring their disappearance in vitiligo-affected areas. These findings reinforce the idea that cell adhesion plays a crucial role in the pathogenesis of the disease.12
InflammasomesInflammasomes are multiprotein complexes that, when activated, trigger a cascade of events resulting in the activation of an enzyme called caspase-1. Inflammasome activation could play a crucial role in the inflammation and destruction of melanocytes in non-segmental vitiligo. Inflammasome activation has been found in vitiligo lesions, associated with increased expression of inflammatory cytokines such as IL-1β and IL-18. Therefore, IL-1β inhibition can be seen as a potential therapeutic target in vitiligo.
Polymorphisms in IL-1β and in NOD-like receptor family pyrin domain containing 1 (NLRP1) inflammasome are associated with an increased risk of developing vitiligo. By recognizing DAMPs, this receptor activates the inflammasome, which, via the caspase-1 pathway, induces the processing of pro-IL-1β into active IL-1β. Although the specific connection between NRLP1 and vitiligo is not completely defined, it is likely that inflammasomes, including NRLP1, play an important role in the inflammatory and cell stress processes that characterize this disease.13,14
Type 1 interferon pathway (IFN type 1)The IFN-1 pathway is an early and transient link in disease progression and marks the junction point between the innate and adaptive immune response. Vitiligo is associated with type 1 interferon activation. Variants of the IFN-induced helicase C domain 1 (IFIH1) gene are related to protection vs vitiligo by inhibiting IFN-α production. This interferon induces chemokines that recruit autoreactive T cells such as CXCL10 and CXCL16. Plasmacytoid dendritic cells (pDC) are responsible for the production of IFN-α in vitiligo skin, stimulated by HSP70.15,36
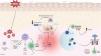
Adaptive immunityDamage to melanocytes caused by oxidative stress activates innate immunity, with consequent cytokine secretion and antigen presentation. This triggers the activation of the adaptive immune system, where autoreactive T lymphocytes exacerbate melanocyte damage in vitiligo-affected skin through the secretion of pro-inflammatory cytokines (Fig. 1).3,4,16,17
Etiopathogenic mechanisms of vitiligo. This figure illustrates all the important keys of how a molecular cascade is triggered, starting with increased melanocyte susceptibility to oxidative stress, which, in the presence of a susceptible genetic basis, leads to immune system activation. CD8+ T cells produce various cytokines such as IFN-γ. The binding of IFN-γ to its receptor activates the JAK-STAT pathway and causes the secretion of CXCL9 and CXCL10. CCL5: chemokine ligand 5; CXCL9: CXC chemokine ligand 9; CXCL10: CXC chemokine ligand 10; CXCL12: CXC chemokine ligand 12; CXCL16: CXC chemokine ligand 16; CXCR3: chemokine receptor type 3; DAMP: damage-associated molecular pattern; IFN-γ: interferon-γ; JAK: janus kinase; MAPK: mitogen-activated protein kinase; NF-kB: nuclear factor kappa light chain enhancer of activated B cells; NLRP1: NOD-like receptor family pyrin domain containing 1 (NLRP1); TNF-α: tumor necrosis factor alpha; ROS: reactive oxygen species; STAT1: signal transducer and activator of transcription 1.
Cytotoxic T lymphocytes (CD8+) are the main immune cells involved in the pathogenesis of the disease, primarily in initial or active phases. The mechanism of action is based on the production of inflammatory cytokines such as TNF-α and IFN-γ, in addition to the release of granzymes and perforins. IFN-γ locally increases levels of CXCL9 and CXCL10 in the skin and serum of vitiligo patients, which in turn attracts pathogenic T cells expressing the CXCR3A receptor.18 A predominant lymphocytic infiltrate of CD8+ T cells has been observed in perilesional skin, correlating with disease activity.3
In vitiligo patients, CD8+ T lymphocytes recognize specific melanocyte antigens (MelanA, tyrosinase, gp100, tyrosinase-related proteins 1 and 2, Mart-1). These antigens are found in significantly higher amounts in the peripheral blood of patients vs healthy individuals. Although this phenomenon is considered the primary pathway of melanocyte destruction in vitiligo, the presence of melanocyte-specific T cells is not sufficient, as they have also been found in healthy individuals, showing an anergic phenotype.19,20
Regulatory T cellsThe imbalance between pro- and anti-inflammatory signaling is crucial in the pathogenesis of vitiligo. The number of regulatory T cells (Tregs), capable of attenuating the immune response, is decreased in patients. Furthermore, their suppressive capacity is also compromised.21
Differences in the immune response in patients with non-segmental vitiligo and healthy individuals have been explored by examining Treg levels and LRP1 receptor expression in monocytes. Treg and LRP1/CD91 levels before and after treatment in vitiligo patients, vs healthy individuals, show higher levels in vitiligo patients, demonstrating that abnormalities in the immune response contribute to the pathogenesis of vitiligo.22
Cytokines and chemokinesCytokines, especially IFN-γ and TNF-α, play a crucial role in melanocyte loss and vitiligo progression. IFN-γ activates the JAK/STAT pathway, and TNF-α activates the MAPK and NF-kB pathways, both contributing to inflammation and cell damage. IFN-γ induces the production of chemokines CXCL9 and CXCL10, which amplify inflammation and immune cell recruitment. In murine models, CXCL10 inhibition prevented the disease and promoted repigmentation. Moreover, CXCL12 and CCL5 play an important role in immune cell recruitment in affected skin, and serum CXCL12 levels are associated with disease activity.23 Additionally, oxidative stress induces the secretion of chemokines like CXCL16.24
Other cytokines elevated in vitiligo include IL-1β, IL-2, IL-17, IL-22, IL-23, and IL-33. However, therapies targeting these molecules have shown contradictory results, underscoring the need for further research to find effective treatments.25–27
AutoimmunityGenetic evidence supports the autoimmune hypothesis as the main mechanism of vitiligo, with approximately 85% of susceptibility genes involved in innate, adaptive immunity, and apoptosis.4,28 This theory is also reinforced by the relationship of vitiligo with other autoimmune disorders, the presence of specific antibodies in patients, and the use of immunomodulatory therapies.4,28
Furthermore, it has been observed that treatments with immune checkpoint inhibitors in cancer patients can induce the development of vitiligo, suggesting a link between autoimmunity and this disease.29
Autoimmune diseases are the main comorbidity associated with vitiligo, including thyroid disorders, pernicious anemia, alopecia areata, connective tissue diseases, among others.30,31
Zombie cells, senescent cells in vitiligoCellular senescence is induced in response to cellular stressors. These cells remain metabolically active and secrete a series of proteins and bioactive factors. It has recently been accepted that the presence of senescent cells contributes to the progression of various diseases, including vitiligo. Thus, a significant subset of melanocytes in vitiligo lesions was found to be positive for p16INK4A vs unaffected skin. This finding suggests that p16INK4A could be involved in regulating melanocyte function in non-segmental vitiligo, possibly as a response to oxidative or inflammatory stressors in the skin microenvironment.32
Trained immunity and immune toleranceA long-term increase in innate memory function, described as trained immunity after vaccination and in other inflammatory diseases, could play a role as an enhancer and continuous trigger in the pathogenesis of vitiligo. After exposure to certain stimuli, the innate immune system is capable of showing an enhanced immunological response to a second stimulus, indicating a memory function of the innate immune system, a concept known as trained immunity. Trained immunity is regulated by epigenetic reprogramming, which includes chemical changes to histones and chromatin accessibility causing sustained changes in the transcription of specific genes.33
Resident memory T cellsThe recurrence of vitiligo in previously affected areas demonstrates a memory response involved in the etiopathogenesis of this disease. This relapse risk could be due to the persistence of tissue-resident memory T (TRM) cells, whose maintenance and function are promoted by IL-15. These TRM cells exhibit high levels of CD122, the IL-15 receptor subunit.34–37 This antibody has demonstrated disease reversal in mice, making it a promising therapeutic target.38 Recently, it has been shown that TRM survival depends on the uptake and metabolism of exogenous fatty acids, suggesting that a possible treatment could be the regulation of lipid metabolism, focused on the elimination of TRMs in peripheral tissues.39
Intrinsic melanocyte abnormalities and epidermal and dermal alterations in vitiligo skinSeveral in vitro and in vivo studies have demonstrated the presence of intrinsic abnormalities in vitiligo melanocytes characterized by increased susceptibility to pro-oxidant agents. In the epidermis of vitiligo lesions, a marked decrease in the expression of c-kit, the stem cell factor receptor, and the MITF transcription factor (microphthalmia-associated transcription factor) has been detected, along with a reduction in endothelin B receptor.40 A marked reduction in microRNA-211 expression has also been observed, associated with a lower oxygen consumption rate, aberrant mitochondrial complexes, alterations in lipid metabolism, and an increase in reactive oxygen species. This microRNA-211 could serve in the future as a biomarker to evaluate the therapeutic response in this disease.41
The role of endoplasmic reticulum (ER) stress in the pathogenesis of vitiligo has been examined due to its connection with the accumulation of misfolded proteins, which activates the unfolded protein response (UPR). This response tries to restore cellular homeostasis, but if it fails, it can contribute to the development of autoimmune diseases. In vitiligo, ER stress could link oxidative stress with autoimmunity. Oxidative stress alters cellular redox potential, extending to the ER and causing accumulation of misfolded proteins. The UPR, crucial for innate and adaptive immunity, is activated in this situation, suggesting its role in regulating and maintaining the immune response in vitiligo.42
Another recent study analyzed the role of mitochondrial energy metabolism in melanocytes from vitiligo patients, finding reduced ATP production, increased proton leakage, altered expression of glycolytic enzymes, and hyperactivity of the PGC1α axis. Furthermore, it was demonstrated that pharmacological stabilization of cardiolipin, a key lipid in the mitochondrial membrane, can reverse the energy dysfunction observed in vitiligo melanocytes, which suggests that manipulating cardiolipin could be a promising new therapeutic strategy for treating vitiligo.43
Vitiligo skin shows morphological changes in the epithelium and in the upper dermis. Histologically, reduced pigmentation in the basal layer is observed, along with an absence of melanocytes in the lesions, which can be confirmed by specific immunohistochemical techniques, such as staining for HMB-45 or S100 proteins. Occasionally, a pattern of associated lichenoid dermatitis is observed, with CD3+, CD8+ T lymphocytes, more evident in perilesional skin. On the other hand, Langerhans cells are increased in the epidermis, the basement membrane is thickened, and cytoplasmic vacuolization phenomena are observed.44,45 It should be noted that Dickkopf 1 (DKK1) protein, involved in reducing melanogenesis, is overexpressed in fibroblasts in lesional skin.46 There is also increased fibronectin expression and reduced elastin in lesional dermis, with no changes observed in collagen fibers.47,48
Genetic changes involved in the etiopathogenesis of vitiligoVitiligo is a complex disease with a significant genetic component, estimated between 75% and 83%. This theory involves multiple genes, most of which correspond to the immune system, with a smaller number involving melanocytes. There is a notable overlap between genes involved in vitiligo and those that have been associated with autoimmune disorders. First-degree relatives of vitiligo patients have a 6–8% risk of developing the disease, and the concordance rate between monozygotic twins is approximately 23%.28,34
Five genome-wide association studies (GWAS) have been conducted on vitiligo in European and Asian populations, identifying at least 54 vitiligo susceptibility loci.49,50
Human leukocyte antigen regionSeveral genes within the human leukocyte antigen (HLA) region, specifically in class I and II regions, are associated with vitiligo. These studies include GWAS association analyses and linkage studies in affected families. The pathogenesis of vitiligo has been associated with the XBP1 gene, a transcription factor that plays a crucial role in endoplasmic reticulum stress response and regulates HLA class II gene expression.51 In the Chinese population, HLA-DQB1 and HLA-B have been identified as associated risk factors; in European populations, 3 specific loci; and 1 in Asians (linked respectively to FOXD3 and PDGFRA).52–54 On the other hand, some studies correlate HLA-A09 and HLA-Aw19 with a lower risk for the disease55 (Table 1).
HLA genes associated with increased risk of developing vitiligo. These studies include genome-wide association studies (GWAS) and linkage analyses in affected families.51–60
| HLA genes |
|---|
| HLA-A02 |
| HLA-Aw31 |
| HLA-A32 |
| HLA-A33 |
| HLA-DQB106 |
| HLA-DQB10303 |
| HLA-DR4 |
| HLA-DRB1*07 |
| HLA-DR7 |
HLA: human leukocyte antigen.
Several genes linked to vitiligo susceptibility related to immunomodulation of innate and adaptive immunity have also been identified (Tables 2 and 3).
Main genes involved in the etiopathogenesis of vitiligo.
| Gene | Chromosome and region | Function |
|---|---|---|
| XBP151 | Chr 22q12 | Transcription factor involved in HLA II expression, cellular stress response |
| FOXD353 | Chr 1p31.3-p32.2 | Transcription factor involved in cell differentiation, immune regulation |
| PDGFRA52 | Chr 4q13-q21 | Transcription factor involved in cell proliferation, and angiogenesis |
| NLRP164 | Chr 17p13 | Pathogen sensing, inflammasome formation, caspase-1 activation, cytokine secretion, and pyroptosis induction |
| PTPN2261 | Chr 1p13.2 | It codes for a tyrosine phosphatase involved in T-cell signaling, which is linked to various autoimmune diseases. |
| IKZF462 | Chr 12q13.2 | FOXP3-mediated gene silencing in Tregs |
| FOXP362 | Chr X | Gene silencing in Tregs |
| DDR165 | Chr 6p21.3 | It codes for a tyrosine kinase receptor involved in the adhesion of melanocytes to the basement membrane. |
| VEGF66 | Chr 6p21.1 | Regulator of angiogenesis involved in chronic diseases and neoplasms |
HLA: human leukocyte antigen.
Immunoregulatory genes involved in T cell development, activation, signaling, and innate immune response associated with vitiligo.4,28,49,50
| T cell development | T cell receptor signaling | T cell activation | Innate immune response | Chemokine and cytokine receptors |
|---|---|---|---|---|
| CD44 | SLA | BTNL2 | IFIH1 | CXCR5 |
| CD80 | PTPN22 | FOXP3 | TICAM1 | CCR6 |
| UBASH3A | IKZF4 | SH2B3 | ||
| CLNK | IL2RA | |||
| CTLA4 |
Variants of the PTPN22 gene have been associated with several autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, making it a gene with a relevant role in the context of autoimmune diseases.61 The IKZF4 and FOXP3 genes, which play a role in gene silencing in Tregs, have also been linked.62
Several genes related to melanocyte function and survival have been invovled in vitiligo (Table 4). The TYR gene, involved in melanin biosynthesis in melanocytes, has been significantly associated with vitiligo in the European population.63 The roles of many susceptibility loci remain unknown, highlighting the lack of understanding in the etiopathogenesis of vitiligo, which continues to be a challenge today. This genetic knowledge highlights the multifactorial nature of vitiligo with a polygenic inheritance pattern, involving a complex interaction between immune system dysfunction and melanocyte dysfunction.
Genes related to melanocyte function and survival.4,28,49,50
| Autoantigen formation | Melanocyte development and survival | Melanocyte adhesion to basement membrane | Cell death during oxidative stress | Immune-induced apoptosis | Apoptosis regulation | Inflammasome activation |
|---|---|---|---|---|---|---|
| PMEL | ZMIZ1 | DDR1 | RNASET2 | GZMB | FGFR1OP | NLRP1 |
| MC1R | ASIP | CDH1 | FASLG | CASP7 | ||
| OCA2 | BCL2L12 | |||||
| TYR | RERE | |||||
| NEK6 | ||||||
| SERPINB9 | ||||||
| BAD |
Oxidative stress plays a fundamental role in the pathogenesis of vitiligo, contributing to the damage and destruction of melanocytes. Oxidative stress refers to an imbalance between the production of reactive oxygen species (ROS) and the body's antioxidant capacity to neutralize them. In vitiligo patients, an excessive accumulation of ROS has been observed in melanocytes, which can damage crucial cellular components, including lipids, proteins, and DNA, leading to their dysfunction and death. Cellular damage caused by oxidative stress can release autoantigens and activate an autoimmune response, contributing to melanocyte destruction.4,28,67,68 Former studies have shown elevated levels of oxidative stress biomarkers, such as malondialdehyde (MDA), and decreased antioxidants, such as superoxide dismutase (SOD) and catalase, in both affected skin and blood of patients. Additionally, certain variants in genes related to antioxidant response, such as the catalase (CAT) gene and the glutathione peroxidase (GPX) gene, have been associated with increased susceptibility to vitiligo.67,69,70
Furthermore, vitiligo has been associated with defective recycling of tetrahydrobiopterin (6BH4), essential for melanin formation, leading to an excess of 7BH4 and inhibition of phenylalanine hydroxylase (PAH). This produces hydrogen peroxide (H2O2), which decreases the activity of the dihydropteridine reductase (DHPR) enzyme.71,72 Furthermore, H2O2 negatively affects acetylcholinesterase (AchE) and xanthine oxidase (XO).73,74
Repair mechanisms and immune system activation in vitiligo patients could reduce the risk of skin cancer, which is attributed to immune activation capable of identifying and eliminating damaged cells, including those with potentially precancerous mutations. Additionally, oxidative stress and autoimmunity in vitiligo create a less favorable microenvironment for the development of skin cancer, including melanoma.75
Environmental agents as disease activatorsEnvironmental factors influence the onset or worsening of vitiligo in susceptible individuals (Table 5). Physical trauma, known as the Koebner phenomenon, can induce the appearance of vitiligo patches in areas of repeated friction, wounds, scars, and pre-existing lesions of contact eczema or psoriasis.76 Exposure to chemicals, such as phenolic derivatives, generates a melanotoxic effect in vitiligo by inducing oxidative stress and causing direct damage to melanocytes. Additionally, tattoos, vaccines, and certain cosmetics can also precipitate vitiligo by altering melanocytes. Heat, derived from procedures such as laser hair removal or pulsed light, can damage the skin and trigger depigmentation.77,78 On the other hand, smoking, with its detrimental effects on skin health and the immune system, can exacerbate the disease.79 Furthermore, numerous drugs, including some immunomodulators and biological agents, can induce or worsen vitiligo.80–83 Moreover, viral infections, such as HCV, HIV, varicella-zoster virus (VZV), and COVID-19, and exposure to allergens suchas dust mites, have been associated with vitiligo outbreaks.84–86 Finally, psychological stress is a known factor that can aggravate the condition, possibly due to its impact on the immune system.87 However, there are no studies that prove a direct association between vitiligo and pollution, specific diets, or alcohol consumption.88–90
Environmental triggers of vitiligo.76–86,88–91
| Environmental agents | |
|---|---|
| Chemicals | Phenolic derivatives (monobenzone, rhododendrol), tattoos, vaccines, cosmetic dyes (paraphenylenediamine) |
| Heat | Laser hair removal, intense pulsed light |
| Trauma | Koebner phenomenon in areas of repeated trauma, friction, wounds, use of face masks, residual lesions from contact eczema, psoriasis, etc. |
| Drugs | Paracetamol (acetaminophen), drugs used in hematologic patients (mogamulizumab, TKIs), dermatologic patients (anti-TNF, dupilumab, secukinumab, imiquimod, diphencyprone), neurologic patients (alemtuzumab, carbamazepine, tolcapone, levodopa), antibiotics (chloroquine, clofazimine), beta-blockers, anti-PD1 drugs (pembrolizumab, nivolumab), anti-CTLA-4 drugs (ipilimumab) |
| Infections | HIV, HCV, herpesviruses (varicella-zoster virus, herpes simplex virus), COVID-19 |
| Smoking | – |
| Psychological stress | – |
TKI: tyrosine kinase inhibitors; HCV: hepatitis C virus; HIV: human immunodeficiency virus.
The neural hypothesis suggests that segmental vitiligo is related to nervous system dysfunction. It is postulated that neurotransmitters or neurochemicals released by nerve endings, specifically substance P, can be toxic to melanocytes, causing their destruction in specific areas.92,93 The segmental distribution of vitiligo patches corresponds to the innervation of certain nerves, supporting this hypothesis.92,93 A study analyzing the presence of VZV in segmental vitiligo skin suggests a possible relationship between the 2. The results indicate the presence of viral particles and related changes in the affected skin, suggesting that VZV could contribute to the development or progression of vitiligo.86
Sometimes, however, segmental vitiligo doesn’t follow a dermatomal distribution, suggesting that neuronal mechanisms aren’t the sole cause. For this reason, genetic mosaicism is currently the most accepted theory to explain this condition, though it has not yet been genetically confirmed.94
New pathogenic pathways and potential new therapeutic targetsThe pathogenesis of vitiligo is multifactorial, involving a complex interaction between genetic predisposition and various environmental factors, oxidative stress, and autoimmune responses. The main challenge in creating pathophysiological models is to integrate these diverse theories and elements.
The discovery of new pathogenic pathways raises potential new therapeutic targets to act upon. Emerging therapeutic strategies include the reduction of reactive oxygen species (ROS), the inhibition of IFN-γ and IL-15 signaling through the JAK-STAT pathway, and the use of anti-IL-15 and anti-CD122. Additionally, the reduction of resident memory T cells and intervention in the NRLP1 pathway by acting on the inflammasome are also promising approaches to treat this disease.
CRediT authorship contribution statementAll authors contributed to the preparation and critical modification of the manuscript and approved the submitted version.
Conflicts of interestNone declared.