We present a series of general and specific recommendations based on pathophysiologic considerations for managing the most common adverse effects of apremilast that lead to treatment discontinuation: diarrhea, nausea, and headache. The recommendations are based on a review of the literature and the experience of a multidisciplinary team of 14 experts including dermatologists, rheumatologists, neurologists, gastroenterologists, pharmacists, and nurses. We propose a series of simple algorithms that include clinical actions and suggestions for pharmacologic treatment.
The adverse effects of apremilast can be managed from a multidisciplinary approach. The purpose of optimizing management is to bring clinical benefits to patients.
En el presente artículo, en base a una revisión de la literatura y su experiencia personal, un equipo multidisciplinar de 14 profesionales sanitarios (incluyendo dermatólogos, reumatólogos, neurólogos, gastroenterólogos, farmacéuticos y enfermeras) ha elaborado una serie de recomendaciones generales y específicas (basadas en la fisiopatología) para el manejo de los efectos adversos secundarios a apremilast que con mayor frecuencia conducen a la suspensión del tratamiento (diarrea, náuseas y cefalea). Se aportan algoritmos sencillos de manejo que incluyen aspectos clínicos de evaluación y sugerencias de tratamiento farmacológico.
Los efectos adversos de apremilast pueden ser abordados desde un punto de vista multidisciplinar y la optimización en su manejo pretende proporcionar un beneficio clínico a los pacientes que los sufren.
Since its approval by the European Commission in 2015, apremilast has been used in the treatment of psoriasis and psoriatic arthritis in adult patients.1–5 The agent is an oral inhibitor of phosphodiesterase 4 (PDE-4) and has a good safety profile with a low incidence of adverse effects; those that do occur are mainly of mild or moderate severity and largely involve the digestive tract.1,2 The incidence of these adverse effects is higher during the first 2 months of treatment and then decreases notably, particularly from the second year of use onwards (Table 1).5,6 They often resolve without need for medical intervention or dose adjustment.7 Although the rate of treatment discontinuations due to adverse effects was less than 2% during clinical development,1,2 a higher rate of apremilast discontinuations has been reported in clinical practice (Table 2).8
Frequency of Adverse Drug Reactions Reported During Long-term Follow-up of Apremilast Treatment.
| Year 1, % | Year 2, % | Year 3, % | Year 4, % | Year 5, % | ||
|---|---|---|---|---|---|---|
| Diarrhea | Psoriasis | 17.3 | 2.3 | 1.7 | NE | NE |
| PsA | 15.7 | 3.8 | 2.7 | 1.2 | 0.5 | |
| Nausea | Psoriasis | 15.7 | 0.8 | 1.5 | NE | NE |
| PsA | 15.0 | 2.1 | 2.3 | 0.7 | 0.3 | |
| Headache | Psoriasis | 6.3 | 0.9 | 1.7 | NE | NE |
| PsA | 10.4 | 3.3 | 2.7 | 2.0 | 0.5 | |
| Tension headache | Psoriasis | 9.0 | 1.2 | 1.2 | NE | NE |
| PsA | NE | NE | NE | NE | NE |
Frequency of Adverse Drug Reactions and Treatment Discontinuation of Apremilast for the Treatment of Psoriasis in Clinical Trials and Clinical Practice.
| Diarrhea | Nausea | Headache | ||
|---|---|---|---|---|
| Frequency of adverse effect, % | CT | 17.8 | 16.6 | 13.1 |
| CP | 16.3 | 14.4 | 15.9 | |
| Treatment discontinuation, % | CT | 1.3 | 1.6 | 0.6 |
| CP | 5.3 | 4.3 | 4.3 |
Abbreviations: CP, clinical practice; CT, combined analysis of clinical trials.
Adapted from Ighani et al.8
In order to assess the strategies available for management of adverse effects that most often lead to treatment discontinuation in patients with psoriasis and/or psoriatic arthritis (diarrhea, nauseas, and headache), a multidisciplinary committee of experts in rheumatology, dermatology, neurology, gastroenterology, hospital pharmacy, and nursing, belonging to 14 Spanish hospitals, conducted a broad review of the literature, with special focus on the general pathophysiology of each adverse effect from the point of view of their particular specialty. After considering this information and personal experience, a series of consensus recommendations were made.
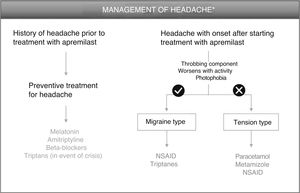
HeadacheDrug-induced headaches are a neurological process triggered by activation of pain-modulating structures in the central or peripheral nervous system. A migraine attack can be triggered through action on different structures involved in its pathophysiology or by altering the antinociceptive structures that are responsible for migraine resolution. In the framework of pathophysiology of migraines, in recent years, the importance of neuropeptides that participate in genesis of pain and other typical symptoms of migraine has been increasingly recognized. Release of neuropeptides leads to nerve fiber sensitization (neuronal hyperexcitability), thereby enhancing central and peripheral pain and inflammation.9 Calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase-activating peptide (PACAP), and their respective receptors are one of the main targets for new migraine therapies.10
Although the mechanism that triggers headache after administration of PDE-4 inhibitors has not been elucidated, it could be related to release of peptides that induce symptoms similar to migraine. The participation of PACAP and other neuropeptides could be responsible for the appearance of these symptoms, such that we might be facing 2 situations: 1) patients with migraine who see their pain exacerbated, whether in intensity or frequency, and 2) patients who have had not suffered from headache and who start with a throbbing headache with sensitization (pathophysiology similar to migraine) or a headache with a different profile.
Patients With History of Headache Before Treatment With ApremilastFor patients with a history of migraine, there is the option of administering preventive treatment 2 weeks before starting apremilast therapy, as later administration may lead to a delay in the resolution of symptoms. Melatonin, at a dose of 3 mg/mg, could be a good option for preventive treatment,11 with triptanes as an option in the event of a migraine attack (Fig. 1, Table 3). Sumatriptan, at a dose of 25–100 mg/day, is indicated for acute treatment of intermittent migraine, with or without aura. If the patient responds after the first dose, and symptoms recur, a second dose could be administered in the following 24 h if more than 2 h have elapsed since the first dose (maximum 300 mg/day). Adverse effects that have been reported frequently for sumatriptan include general disorders (weakness, fatigue, pressure) and musculoskeletal disorders (heaviness, myalgia), which are generally transient and of mild or moderate intensity. Other reactions include dyspnea, increased blood pressure, dizziness, somnolence, and sensory abnormalities. In the event of lack of response to sumatriptan, treatment with paracetamol or nonsteroidal anti-inflammatory drugs (NSAIDs) is recommended.12
Algorithm for management of headache induced by apremilast.
*As an alternative to these treatments, lengthening the initial escalation regimen of apremilast by 1–2 weeks and/or lowering the dose (30 mg/day) can be considered. This strategy may help lower the rate of headache observed in the early phases of treatment.
Management of Headache, Diarrhea, and Nausea Caused by Apremilast Treatment.
| Prior Considerations | Preventive Treatment | Pharmacological Treatment |
|---|---|---|
| Headache | Only in case of headache prior to treatment | Migraine type: |
| Patients with history of headache before starting apremilast treatment |
|
|
| Tension type: | ||
| ||
| Diarrhea |
|
|
| Nausea |
|
|
| Relationship with headache caused by apremilast treatment |
Alternatively, other preventive therapies include amitriptyline or beta-blockers, depending on the profile of the patient. The initial recommended dose of amitriptyline is 10–25 mg, before going to bed, with an increase of 10–25 mg every 3–7 days, depending on the tolerance of the patient (maximum 25–75 mg/day). The analgesic effect is usually felt after 2–4 weeks of treatment, and the most frequent adverse effects include: aggression, somnolence, dizziness, headache, blurry vision, palpitations, tachycardia, constipation, nausea, dry mouth, weight gain, nasal congestion, hyperhidrosis, and orthostatic hypotension.13 Of the beta-blockers, propranolol is indicated for prophylactic treatment of migraine with an initial dose of 40 mg 2 or 3 times a day, with increments by the same amount over weekly intervals depending on the response of the patient up to 80–160 mg/day (maximum 240 mg/day). It should be remembered that administration of propranolol may cause fatigue, bradycardia, cold hands and feet, Raynaud phenomenon, and sleep disorders.14 It should also be remembered that beta-blockers, and propranolol in particular, belong to the group of drugs able to induce or exacerbate psoriasis. A careful assessment of the benefit/risk profile is therefore important prior to recommending its use.
Patients With Headache That Presents After Starting Treatment With ApremilastIn patients who present with headache after administration of apremilast, 3 differential characteristics of the type of headache should be considered: 1) the throbbing component; 2) whether it worsens with activity and/or head movement; and 3) if photophobia is present (Fig. 1). The presence of these 3 components corresponds to a profile of meningeal inflammation denoted migraine type. Therapeutic intervention is only necessary when the headaches have an impact on the quality of life of the patient. In this case, treatment for this type of headache is based on administration of NSAIDs, such as for example naproxen and ibuprofen, or triptanes (Table 3). As a general regimen, daily doses of naproxen range from 550 mg to 1.1 g, with a recommended starting dose of 550 mg followed by 275 mg every 6–8 h, depending on the intensity of the headache.15 The daily recommended dose of ibuprofen is 1.2–1.8 g, administered over several doses and without exceeding a dose of 2.4 mg/day.16 In contrast, if the headache is of the tension type, treatment with paracetamol or metamizole is recommended, with the additional use of an NSAID also an option. A regimen of 500 mg of paracetamol every 4–6 h or 1 g every 8 h is recommended, without exceeding the maximum dose of 3 g/day.17 Metamizole can be administered at a dose of 575 mg (a capsule) 3 or 4 times a day for a week, without exceding the maximum dose of 6 capsules/day. Reactions of hypotension can present during or after treatment with metaizol.18
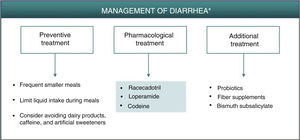
DiarrheaDiarrhea caused by PDE-4 inhibitors is considered of a secretory nature.6 The increase in intracellular cAMP caused by PDE-4 inhibition can trigger intestinal secretion through activation of chlorine channels in the apical membrane of the enterocytes and inhibition of NaCl absorption.6,19 Different isoforms of PDE-4 interact with the cystic fibrosis transmembrane conductance regulator (CFTR), a chlorine channel implicated in secretion and homeostasis, whose hyperactivation may give rise to secretory diarrhea.20 Through protein kinase A (PKA), cAMP also promotes intestinal secretion by activation of different membrane proteins in the enterocytes, such as K+ and anion channels and NaK2Cl transporters.19 In addition, it has been suggested that early resolution of symptoms associated with diarrhea caused by apremilast (in approximately 2 weeks) could be due to upregulation of other PDEs in the intestine, as a compensatory mechanism for PDE-4 inhibition.7
As described for headache, the best strategy for appropriate management of gastrointestinal adverse effects, when considered necessary, is to administer a treatment that is thought to act effectively on the underlying pathophysiology. Preventive measures such as administration of apremilast during meals, avoiding excessive liquid intake or intake of caffeine and sweeteners could reduce or delay the onset of symptoms of secretory diarrhea caused by apremilast.7 Although not fully accepted,21 it is possible that administration of probiotics could reduce the symptoms associated with gastrointestinal intolerance, thus permitting better therapeutic compliance and a lower discontinuation rate. On the other hand, fiber supplements and administration of bismuth subsalicylate may be used as a complementary therapy to reduce water loss and the number of depositions (Fig. 2, Table 3).22 In any case, it is recommended to follow a simple treatment regimen that is not accompanied by major changes in the patient’s lifestyle.
Algorithm for management of diarrhea induced by apremilast.
*As an alternative to these treatments, lengthening the initial escalation regimen of apremilast by 1–2 weeks and/or lowering the dose (30 mg/day) can be considered. This strategy may help lower the rate of diarrhea observed in the early phases of treatment.
Apremilast-induced diarrhea is usually self-limiting and resolves in the first few weeks of treatment. If pharmacological intervention is deemed necessary, secretory diarrhea could be treated effectively with racecadotril, an antisecretory agent that can be used for the treatment of apremilast-induced diarrhea (Fig. 2, Table 3). Racecadotril acts by inhibiting intestinal encephalinase,23 thus reducing water and electrolyte secretion to the intestinal lumen.24 Administration of 100 mg every 8 h, preferably before meals, has been shown to shorten the duration of diarrhea symptoms, reduce the number of depositions, and reduce the need for rehydration.25 This regimen is recommended to continue for 7 days, until attaining 2 depositions/day. Adverse skin reactions, generally moderate, have been reported, along with headache and cases of hypersensitivity/angioneurotic edema in patients in treatment with racecadotril.26 Other pharmacological options for the treatment of diarrhea caused by apremilast include loperamide and codeine. Loperamide is indicated for treatment of acute diarrhea with an initial dose of 4 mg (2 tablets), continuing with 1 tablet after each deposition, for a maximum of 2 days and 4 tablets/day. Gastrointestinal disorders (nausea and dysgeusia) have often been reported after treatment with loperamide. If clinical improvement is not observed in 48 h, or if symptoms of constipation, ileus, or abdominal distension appear, administration of loperamide should be discontinued.27 In the case of treatment with codeine, the recommended regimen is 1 tablet (21.4 mg) every 6 h; this can be increased to 2 tablets per administration, for a maximum duration of 2 days, administering 6 tablets/day. Administration of codeine can cause somnolence and alcohol intake should be avoided during treatment, as this can enhance the depressor effect on the CNS.28
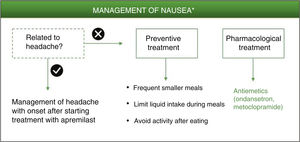
NauseaNausea induced by PDE-4 inhibitors appears to be triggered by central and peripheral mechanisms.6,29 This adverse effect has been associated with lack of selectivity of PDE-4 inhibitors for the different isoforms (A, B, C, D) expressed in different cell types.29,30 Specifically, the PDE-4 D isoform is expressed in neurons of the area postrema, where the chemoreceptor trigger zone that links with the vomit center is located.3 The increase in intracellular levels of cAMP in neurons of the area postrema ultimately triggers the emetic response.30 While apremilast has been reported to be highly specific for PDE-4, it has a similar potency for each of the different isoforms of this enzyme.3,31,32 The lack of selectivity for the PED-4 isoform could explain the improved therapeutic index of apremilast in in vivo models compared with other PDE-4 inhibitors for gastrointestinal adverse effects.3
Among these nonpharmacological interventions, frequent smaller meals as well as limited liquid intake during meals may help reduce the sensation of nausea.7 As with the preventive measures for diarrhea, a simple and personalized approach is recommended according to the patient’s characteristics, without limiting his or her quality of life.
Ondansetron is a potent and highly selective serotonergic 5HT3 antagonist that blocks the vomiting reflex at both the central and peripheral level. It is therefore indicated for controlling nausea and vomiting following surgery or induced by radiotherapy or cytotoxic chemotherapy.33 Given its high efficacy as an antiemetic and its good tolerability, ondansetron could be considered a good pharmacological option for treatment of nausea that persists during treatment with apremilast (Fig. 3, Table 3). Ondansetron can be administered orally or intravenously, with the oral regimen being 8 mg between 1 or 2 h prior to emetogenic treatment, followed by 8 mg every 12 h for up to a maximum of 5 days. The most frequently reported adverse effects are headache, burning sensation (hot flushes), and constipation.33 It should be remembered that ondansetron is not currently reimbursed under the Spanish National Health System, and so its use would require an off-label request. Alternatively, metoclopramide could be used. This is a neuroleptic agent with antiemetic properties used for prevention and symptomatic treatment of nausea and vomiting.34 A single dose of 10 mg of metoclopramide is recommended. This dose can be repeated up to 3 times a day, with at least 6 h between dosing (even in the case of vomiting or dose rejection) up to maximum duration of 5 days. During treatment with metoclopramide, particularly when used at high doses, adverse effects such as diarrhea, asthenia, somnolence, extrapyramidal disorders, parkinsonism, akathisia, depression, and hypotension may appear.34
Algorithm for management of nausea induced by apremilast.
*As an alternative to these treatments, lengthening the initial escalation regimen of apremilast by 1–2 weeks and/or lowering the dose (30 mg/day) can be considered. This strategy may help lower the rate of nausea observed in the early phases of treatment.
The possibility should also be considered that nausea is not a direct result of apremilast treatment but rather a side effect of the neurological adverse effects described above. It is therefore recommended to evaluate each case individually, and tailor management of the adverse effect that has triggered the symptoms in the first place. Metoclopramide is also indicated for the treatment of nausea and vomiting induced by acute migraine, and can be administered in combination with oral analgesics to improve absorption of these analgesics.34
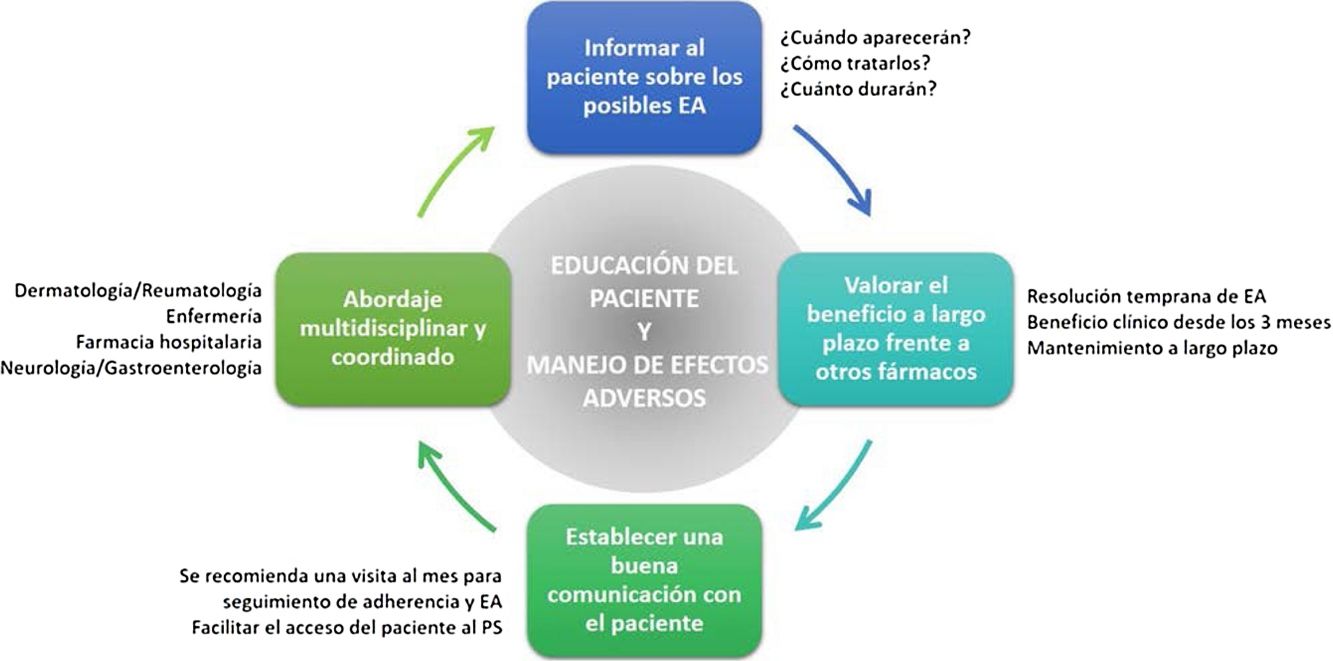
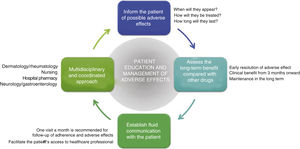
General RecommendationsTaking into consideration the above, a series of general recommendations are derived to facilitate the prevention and management of possible adverse effects caused by apremilast in clinical practice (Fig. 4, Table 4).
Key Points and General Recommendations in the Prevention and Management of the Main Adverse Effects Caused by Apremilast (Fig. 4).
|
|
|
|
|
|
|
|
|
Adverse effects caused by apremilast should be managed in a multidisciplinary setting. Optimization of their management would no doubt be of clinical benefit to patients affected by these adverse effects.
Conflicts of InterestEsteban Daudén: advisory board member, consultant, grant recipient, research support, participation in clinical trials, speaker fees with the following pharmaceutical companies: Abbott/AbbVie, Almirall, Amgen, Biogen, Celgene, Janssen-Cilag, Leo Pharma, Lilly, MSD, Novartis, and Pfizer.
Jorge Alonso Suárez: advisory board member, consultant, grant recipient, research support, participation in clinical trials, speaker fees with the following pharmaceutical companies: AbbVie, Almirall, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, Celgene, Lilly.
M. Carmen Herrero Manso: consultancy, courses, book chapters, or congress attendance with UCB Pharma, Celgene, MSD, Pfizer, Mylan, BMS, AbbVie, Janssen, Sandoz, Gedeon Richter, Novartis, Lilly, Gebro, Nordic.
Ignacio Marín-Jiménez: consultancy, support for investigations or congress attendance with: AbbVie, Amgen, Celgene, Dr. Falk Pharma, Faes Farma, Ferring, Fresenius, Janssen, MSD, Pfizer, Sandoz, Takeda, and UCB-Pharma.
M. Dolores Martín-Arranz: Advisory Board member and support for investigation with the following pharmaceutical companies: MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Shire Pharmaceuticals, Tillotts Pharma, Faes Farma.
Miguel A. Rodríguez-Sagrado: renumerated activities for AbbVie, Biogen, Celgene, Gilead, Intercept, Janssen, Merck-Serono, MSD, Novartis, Roche, Sandoz, Tesaro, and ViiV.
José Rosas Gómez de Salazar: Advisory Board member: AbbVie, Celgene, Grifols, Janssen, Lilly, Pfizer. Speakers fees: Amgen, Grifols, Janssen, Lilly, MSD, Novartis, Pfizer, Stada
Laura Salgado-Boquete: advisory board member, consultant, grant recipient, research support, participation in clinical trials, speaker fees from the following pharmaceutical companies: AbbVie, Almirall, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, Lilly.
The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Daudén Tello E, Alonso Suárez J, Beltrán Catalán E, Blasco Maldonado C, Herrero Manso MC, Jiménez Morales A, et al. Manejo de los efectos adversos de apremilast desde un abordaje multidisciplinar. Actas Dermosifiliogr. 2021;112:134–141.