Mucosal melanoma is a rare melanoma subtype that differs from the cutaneous form of the tumor in its biology, clinical manifestations, and management. Diagnosis is usually late due to a lack of early or specific signs and the location of lesions in areas that are difficult to access on physical examination. Surgical excision is the treatment of choice for localized disease. The value of sentinel lymph node biopsy and lymphadenectomy is still unclear. Radiotherapy can be used as adjuvant therapy for the control of local disease. KIT mutations are more common than in other types of melanoma and this has led to significant advances in the use of imatinib for the treatment of metastatic mucosal melanoma.
El melanoma mucoso es un subtipo infrecuente de melanoma que difiere del melanoma cutáneo en su biología, clínica y manejo. El diagnóstico suele realizarse de forma tardía debido a su localización en zonas de difícil acceso a la exploración física y a la falta de signos específicos y tempranos. La cirugía es el tratamiento de elección en caso de enfermedad localizada. El papel de la biopsia selectiva de ganglio centinela y de la linfadenectomía permanece todavía incierta. La radioterapia se puede emplear como tratamiento adyuvante con el fin de controlar localmente la enfermedad. Existe un mayor porcentaje de mutaciones en KIT que en otros tipos de melanoma, lo que ha llevado a avances significativos en el tratamiento de la enfermedad metastásica con imatinib.
Mucosal melanoma arises from melanocytes in the mucosal membranes. Melanocytes are found on all mucosal surfaces, where rather than protect against sun damage, they have immunological, antibacterial, and phagocytic functions and participate in antigen presentation and cytokine production.1 While all melanocytes have the same embryonic origin, the microenvironment to which they are exposed varies according to their final destination. Depending on their location, they will be found in different types of tissues, surrounded by different cells, and as a result, their growth and maintenance—and consequently the development of melanoma—will be affected by different adhesion molecules and signaling pathways.2
Because mucosal melanoma is rare, there are no specific staging or treatment protocols, and much remains to be learnt about the pathogenesis of this disease.
EpidemiologyMucosal melanoma accounts for 1% of all melanomas, but in contrast to cutaneous melanoma, whose incidence is rising, the incidence of mucosal melanoma has remained stable.3–5 This subtype of malignant melanoma affects first and foremost the head and neck, followed by the anorectum and the vulva and vagina.
Mucosal melanoma has a later onset than the cutaneous form. The mean age at diagnosis is 70 years,6 although melanoma of the oral cavity develops sooner.7 Unlike cutaneous melanoma, mucosal melanoma is more common in women, with a male to female ratio of 1.85:1.3 This higher frequency in women can be explained by the relatively high frequency of melanoma of the female genital tract, which is the most common type of melanoma in women.8 The head and neck is the most common site of mucosal melanoma in men.
Blacks, Asians, and Hispanics have a higher proportion of mucosal melanoma than other types of melanoma.9 Up to 9% of all melanomas diagnosed in blacks and Asians are mucosal, compared with 1% in whites.8 The absolute incidence of mucosal melanoma, however, is higher in whites.
Etiology and PathogenesisBecause mucosal melanoma is a rare condition, little is known about its pathogenesis, and no risk factors have been identified to date. Unlike cutaneous melanoma, mucosal melanoma is not associated with UV radiation exposure. Furthermore, no associations have been observed with human papillomavirus, herpesvirus, or polyomavirus.2 Formaldehyde has been postulated as a risk factor for sinonasal melanoma,10,11 and melanoma of the oral cavity may be preceded by oral melanosis.12 While tobacco can induce pigmented lesions on the oral mucosa, there is insufficient evidence to conclude that this substance is carcinogenic in mucosal melanoma.
Different types of melanoma are associated with different mutations. BRAF mutations, for instance, are common in cutaneous melanoma but rare in mucosal melanoma.13 A higher proportion of mutations and multiple copies in the receptor tyrosine kinase gene KIT have been found in mucosal melanoma, with figures ranging from 15.6% to 39%, depending on the series.14,15 Beadling et al.15 detected KIT mutations in 15.6% of mucosal melanomas and increased KIT copy number in 26.3%. The authors also reported that these percentages varied according to the site of the melanoma, with higher rates observed for tumors in the vulvar and vaginal regions (44.4%) than in the head and neck (8.3%). In a similar study, KIT mutations were detected in 35% of vulvar melanomas, 9% of anorectal melanomas, 7% of nasal cavity melanomas, 20% of penile melanomas, and 0% of vaginal melanomas.16NRAS mutations were also detected in 10% of the mucosal melanomas analyzed and BRAF mutations in 6%. One European study reported KIT mutations in 30% of genital melanomas, but did not detect similar mutations in sinonasal or anal lesions.17 Other genetic alterations described for mucosal melanoma are a higher frequency of focal amplifications of CDK4 and loss of CDKN2A locus, as well as chromosomal aberrations distinct to those seen in melanomas arising in skin with chronic sun damage.18 The studies published to date have analyzed few cases, and larger studies are therefore needed to determine whether the tendencies described above are significant. That said, the fact that genetic mutations vary among different types of melanoma suggests that melanoma subtypes differ not only clinically but also biologically.
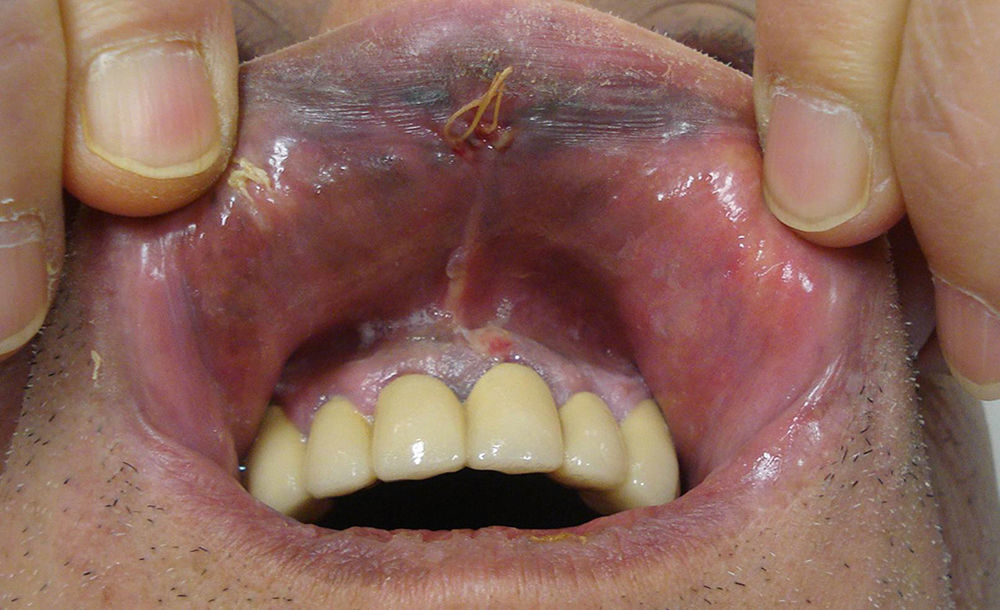
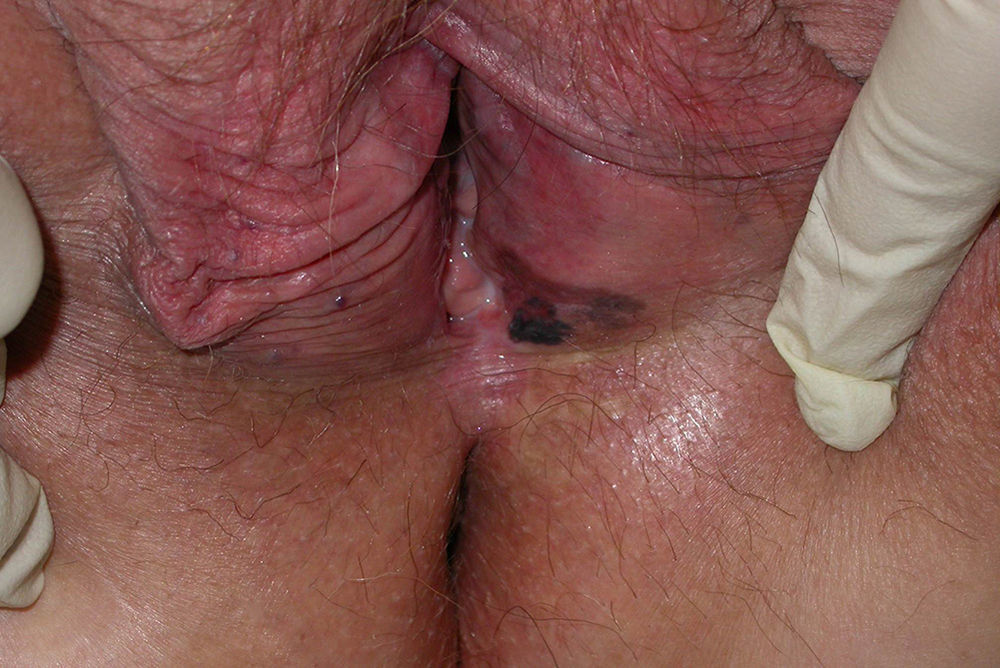
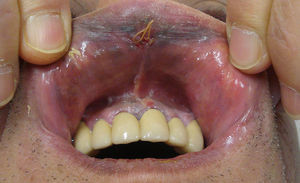
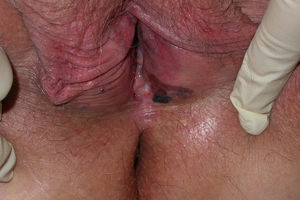
Clinical Characteristics and DiagnosisMucosal melanoma is difficult to diagnose because of its highly variable clinical presentation and its location in areas that are difficult to access during physical examination (Figs. 1 and 2). It is frequently confused with other conditions for a long time, and is often at an advanced stage by the time diagnosis is confirmed by biopsy.
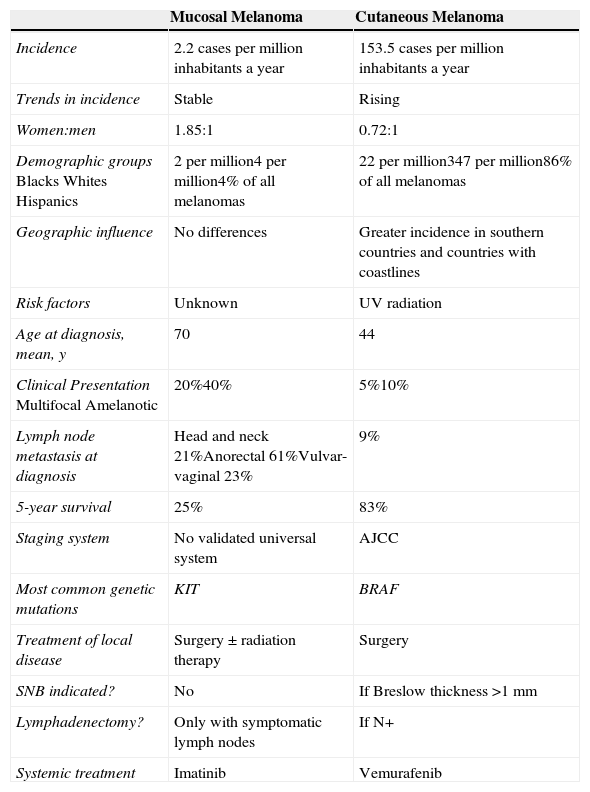
Approximately 20% of mucosal melanomas are believed to be multifocal19 and 40% amelanotic,20 compared with just 5% and 10% for cutaneous melanomas, respectively (Table 1).
Comparison Between Mucosal and Cutaneous Melanoma.
| Mucosal Melanoma | Cutaneous Melanoma | |
|---|---|---|
| Incidence | 2.2 cases per million inhabitants a year | 153.5 cases per million inhabitants a year |
| Trends in incidence | Stable | Rising |
| Women:men | 1.85:1 | 0.72:1 |
| Demographic groupsBlacksWhitesHispanics | 2 per million4 per million4% of all melanomas | 22 per million347 per million86% of all melanomas |
| Geographic influence | No differences | Greater incidence in southern countries and countries with coastlines |
| Risk factors | Unknown | UV radiation |
| Age at diagnosis, mean, y | 70 | 44 |
| Clinical PresentationMultifocalAmelanotic | 20%40% | 5%10% |
| Lymph node metastasis at diagnosis | Head and neck 21%Anorectal 61%Vulvar-vaginal 23% | 9% |
| 5-year survival | 25% | 83% |
| Staging system | No validated universal system | AJCC |
| Most common genetic mutations | KIT | BRAF |
| Treatment of local disease | Surgery ± radiation therapy | Surgery |
| SNB indicated? | No | If Breslow thickness >1mm |
| Lymphadenectomy? | Only with symptomatic lymph nodes | If N+ |
| Systemic treatment | Imatinib | Vemurafenib |
Abbreviations: AJCC, American Joint Committee on Cancer; SNB, sentinel lymph node biopsy.
In patients with focal oral pigmentation, the main considerations in the differential diagnosis should be melanotic macule and smoker's melanosis. Melanotic macule is a small, well-demarcated, brown-black homogeneous lesion generally located on the lips or gums. Smoker's melanosis, in turn, presents as multiple macules that coalesce to form a brown discoloration on the labial surface of the lower gum; it affects between 25% and 31% of smokers. It may disappear several months after the patient quits smoking. In certain cases, the differential diagnosis should also include causes of more diffuse oral mucosal pigmentation, such as hormones, medication, postinflammatory reactions, physiological pigmentary changes, and foreign bodies.21
The differential diagnosis in patients with focal genital pigmentation should include genital melanotic macule, which is a small, homogeneous lesion that remains unchanged over time. Biopsy is warranted if the lesion changes or displays atypical characteristics. The presence of multiple spots or more diffuse pigmentation in this area requires exclusion of systemic disease, such as lentiginosis or a hormonal disorder.22
Dermoscopy shows a multicomponent pattern in 75% of cases and a homogeneous pattern in 25%. The algorithms used for the dermoscopic diagnosis of cutaneous melanoma are also valid and offer high sensitivity and specificity for mucosal melanoma.23 Combinations of blue, gray, and white areas are indicative of mucosal melanoma.24
When a diagnosis of mucosal melanoma is established, it is essential to rule out regression and cutaneous or eye melanoma metastasis.2 Skin and eye examination are mandatory in patients with no previous history of melanoma. Detection of in situ melanoma has an important role in distinguishing between primary and metastatic melanoma.25
Locoregional lymph node metastasis is found in 9% of cutaneous melanomas at the time of diagnosis. Nodal involvement at the time of diagnosis, however, is more common in mucosal melanoma, with rates of 21% described for head and neck melanoma, 61% for anorectal melanoma, and 23% for vulvar-vaginal melanoma.7
StagingThere is no universal staging system for mucosal melanoma. The systems used vary according to the location of the melanoma and are the same as those used for other tumors at the same site.
There is, however, a simplified staging system, described by Ballantyne,26 that can be applied to all types of mucosal melanoma:
Stage I: Localized disease
Stage II: Regional lymph node involvement
Stage III: Distant metastasis
Because mucosal melanoma is so rare, it has not been evaluated in randomized controlled trials, and clinical practice is therefore based on data from case series and retrospective analyses.
If the disease is localized at the time of diagnosis, the goal of management should be to achieve locoregional control through surgery with or without radiation therapy. The treatment of choice in all cases is surgical excision with free margins. When disease-free margins are not achieved, or when excision is unfeasible, the treatment of choice is radiation therapy with adjuvant or palliative intent. Local control is difficult to achieve in mucosal melanoma because of the complicated location and multifocal nature of the lesions. A field defect is believed to exist in which multiple primary lesions may be present at the time of diagnosis or develop during follow-up.19 Multifocal lesions appear to be more common in mucosal melanoma of the vulva, the male urethra, and the head and neck.27–29 The presence of multiple lesions suggests the proliferation of atypical melanocytes within the mucosa, posing a challenge for local control.
The aim of aggressive locoregional management with surgery and radiation therapy is to achieve local control, and it is therefore necessary to assess each case individually and weigh up the benefits and risks. The role of SNB and elective lymphadenectomy is still not clear in this setting.
Mucosal Melanoma of the Head and NeckWithin the head and neck area, mucosal melanoma is more common in the nasal cavity (59%-80%) than in the oral cavity (16%-41%).29,30 Disease is localized at the time of diagnosis in most cases (80%) thanks to the appearance of early symptoms, such as epistaxis, nasal obstruction, visual changes, and oral discomfort. Lymph node metastasis at the time of diagnosis is more common in oral melanomas than sinonasal melanomas (25% vs 6%).29 Histologic vascular invasion, a tumor thickness of over 5mm, and more advanced disease at diagnosis have all been associated with worse prognosis.29 Overall prognosis is poor in head and neck mucosal melanoma, with 2-year and 5-year survival rates of 26% and 8%, respectively.30
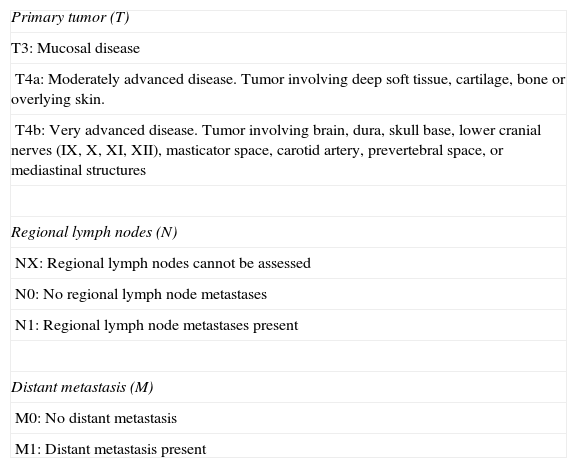
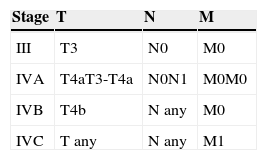
The American Joint Committee on Cancer (AJCC) cancer staging system31 is used to stage disease, and the fact that local disease is directly considered stage III reflects the poor prognosis of mucosal melanoma of the head and neck. Advanced disease is classified as stage IV, which is further stratified into stages IVA, IVB, and IVC, depending on the level of local disease and the presence of lymph node or distant metastasis (Tables 2 and 3).
TNM Classification of Mucosal Melanoma of the Head and Neck.
| Primary tumor (T) |
| T3: Mucosal disease |
| T4a: Moderately advanced disease. Tumor involving deep soft tissue, cartilage, bone or overlying skin. |
| T4b: Very advanced disease. Tumor involving brain, dura, skull base, lower cranial nerves (IX, X, XI, XII), masticator space, carotid artery, prevertebral space, or mediastinal structures |
| Regional lymph nodes (N) |
| NX: Regional lymph nodes cannot be assessed |
| N0: No regional lymph node metastases |
| N1: Regional lymph node metastases present |
| Distant metastasis (M) |
| M0: No distant metastasis |
| M1: Distant metastasis present |
Source: AJCC cancer staging manual, 7th ed. (2010).
The treatment of choice for stage III and IV disease is surgery, with a preference for endoscopic procedures to minimize morbidity. Complete resection with free margins is difficult to achieve in mucosal melanoma of the head and neck due to its lentiginous growth pattern, its multifocal nature, and its complicated anatomic location. Recurrence is common (29%-79%), even with aggressive surgery.29 When local recurrence is detected, restaging is necessary before surgery is repeated, as local recurrences are associated with distant metastasis.32
The role of SNB is also unclear in mucosal melanoma of the head and neck,33 and there is insufficient evidence to show that adjuvant interferon alfa-2b therapy improves survival following a positive SNB. Lymph node dissection is only recommended in the case of clinically enlarged lymph nodes. Survival is similar in patients with and without lymph node involvement because of the high rate of early hematogenous spread, even in patients who have undergone aggressive treatment of lymph node metastases.34 Radiation therapy has been used as adjuvant therapy following surgical excision, but its benefit is unclear and it does not improve survival.35–37 It can be used to improve local control.
Anorectal Mucosal MelanomaAnorectal mucosal melanoma affects the anal canal, the rectum, and intermediate sites in equal proportions.38 It is found with greater frequency in women, but this could be due to a confounding effect as women undergo perineal evaluation more frequently during gynecological examinations.5 The most common symptoms are bleeding, pain, and prolapse. Polypoid melanoma, with or without pigmentation, is common. Hemorrhoids, polyps, and adenocarcinoma are the main diagnostic clues.
At the time of diagnosis, 60% of patients with anorectal mucosal melanoma have lymph node metastasis and 20% have distant metastasis.7 Prognosis is poor, with a 5-year survival of 20% in the case of resectable locoregional disease and 0% in the case of advanced disease.39
The simplified Ballantyne system is used for staging.26 Perineural invasion on histology has been identified as an independent predictor of mortality.6
The treatment of choice for local disease is surgery. Wide yet conservative margins are preferred, as more aggressive surgery results in higher morbidity without improving prognosis.39–41 Prognosis is not improved by SNB or lymphadenectomy, and lymph node metastases are not associated with a higher frequency of recurrence or worse prognosis.40 Only symptomatic lymph nodes should be removed. Adjuvant radiation therapy can help to control local disease but it does not improve prognosis.
Vulvar-Vaginal Mucosal MelanomaMost cases of mucosal melanoma affecting the female genital tract are found in the vulva, with under 5% of cases observed in the vagina. Vulvar melanoma has a better prognosis (5-year survival of 50% vs 19% for vaginal melanoma) and affects older patients (60-80 vs 50-70 years).42–44 Melanoma of the vulva is the second most common malignancy in this location after squamous cell carcinoma, and it is found most frequently in the clitoral area and the labia majora. It can present in the form of bleeding, a vulvar mass, itching, or dysuria. Lesions may be amelanotic or accompanied by satellite lesions.31
Ballantyne's simplified staging system is also used to stage vaginal melanoma.26 The AJCC has a specific staging system for vulvar melanoma,31 and AJCC stage is considered the best predictor of survival in this subtype of melanoma.
Excision with wide margins is indicated for locoregional disease; aggressive surgery is avoided as it does not improve survival.45,46 As in other subtypes of mucosal melanoma, the role of SNB is unclear, and lymph node dissection is only indicated for symptomatic lymph nodes.
Mucosal Melanoma in Less Common LocationsA small number of mucosal melanoma cases have been reported in other less common sites such as the male urethra, the bladder, the esophagus, and other parts of the intestine.47–51
Adjuvant TherapyThe activity of interferon alfa is unknown in mucosal melanoma, which is considered to be biologically distinct from other forms of melanoma and has not been investigated in clinical trials due to its high resistance to systemic treatments. It has also been excluded from recent clinical trials comparing adjuvant high-dose interferon alfa with ipilimumab and ipilimumab with placebo.
Adjuvant radiation therapy has been used to improve local control of disease following surgical excision, but its benefit is unclear, as no improvement in survival has been observed.36–38
Systemic Treatment for Advanced Mucosal MelanomaStandard chemotherapy with dacarbazine has shown limited efficacy in cutaneous melanoma and appears to be even less effective in mucosal melanoma.52 A number of small single-center studies have reported low partial and complete response rates for a more aggressive biochemotherapy regimen consisting of cisplatin, vinblastine, dacarbazine, interferon alfa-2b, and interleukin 2 in patients with various subtypes of mucosal melanoma.53–55 Based on results from these small series, the response rates would appear to be similar to those seen in cutaneous melanoma.
There is a phase II randomized clinical trial underway comparing observation versus interferon alfa-2a versus chemotherapy (temozolomide plus cisplatin) in patients with resected mucosal melanoma. The results to date show improved overall and disease-free survival in the chemotherapy arm.56
Ipilimumab was the first agent to show a beneficial effect in terms of improved overall survival in patients with advanced cutaneous melanoma in a phase III trial.57 Data, however, are still lacking on the use of ipilimumab in mucosal melanoma.
The generation of new knowledge on genetic alterations in different types of melanoma has led to the development of agents with new therapeutic targets. In the case of mucosal melanoma, small molecule KIT inhibitors, such as imatinib, have demonstrated activity in patients with KIT mutations and/or amplifications58 In one study of 13 patients with mucosal melanoma and KIT alterations treated with imatinib, 1 patient (mutation and amplification) exhibited complete lasting response, another (mutation but not amplification) exhibited partial lasting response, and another (mutation and amplification) exhibited transient partial response.59 Not all KIT mutations are oncogenic. Tumors with mutations in exons 11 and 13 are particularly sensitive to KIT inhibition. A response rate of 23% was observed in a phase II trial of 43 patients harboring KIT mutations treated with imatinib.60 The study included 11 patients with mucosal melanoma, but no subgroup analysis was performed for clinical response. Another study currently underway is investigating the use of dasatinib in patients with different types of melanoma and KIT mutations.
The development of early resistance has been observed in patients who initially respond to KIT inhibitors. While the mechanism of resistance is not clear, it might be related to the appearance of new, distinct, mutations. In one study, for instance, a patient developed a previously undetected NRAS mutation,61 and in another, upregulated mammalian target of rapamycin (mTOR) activity was detected in a patient who responded well to everolimus.62 Additional studies are needed to determine these mechanisms of escape and potential targets for new treatments or combinations of treatments. A clinical trial is currently investigating the use of nilotinib in patients who are resistant to or intolerant of another tyrosine kinase inhibitor (NCT00788775).
Vemurafenib would appear to be indicated in patients with mucosal melanoma, as there do not appear to be differences in response to treatment between patients with mucosal melanoma harboring BRAF mutations and patients with other types of melanoma.63
It would be interesting to conduct a study of KIT and BRAF mutations in patients with advanced mucosal melanoma. Should any of these mutations be detected, these patients should be considered candidates for inclusion in clinical trials with KIT inhibitors (imatinib, sunitinib, or nilotinib) or BRAF kinase inhibitors (vemurafenib). If these patients developed new mutations, additional molecular studies should be considered, If no further mutations were detected, treatment with ipilimumab could be useful.5
ConclusionsMucosal melanoma differs both clinically and biologically from cutaneous melanoma. It tends to be diagnosed late and has a poor prognosis. Surgical excision with free margins is generally difficult because of the complicated location of the tumors and the multifocal nature of the disease.
Surgery is the treatment of choice for local disease, and the aim is to achieve disease-free margins without radical excision. The role of SNB and elective lymphadenectomy remains unclear. Radiation therapy can be used as adjuvant treatment to help achieve local control. No improvement in prognosis has been found after radical surgery, SNB, lymphadenectomy, or radiation therapy.
The discovery of KIT mutations has spawned enormous therapeutic advances in mucosal melanoma, although a greater understanding is needed of resistance to KIT inhibitors and possible therapeutic targets. More studies are therefore needed to improve the prognosis of mucosal melanoma.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ballester Sánchez R, de Unamuno Bustos B, Navarro Mira M, Botella Estrada R. Actualización en melanoma mucoso. Actas Dermosifiliogr. 2015;106:96–103.