Merkel cell carcinoma (MCC) is a rare, fast-growing, aggressive neuroendocrine tumor that frequently causes locoregional recurrence and distant metastasis. It tends to affect sun-exposed areas (mainly the head, neck, and upper extremities) and associated risk factors include immune suppression, elderly age, fair skin, and a history of prior malignancy.1 Imaging modalities used to stage disease and plan surgery include computed tomography and magnetic resonance imaging,2,3 but very few studies have described the ultrasound features of MCC.
We present the ultrasound findings of 5 cases of primary MCC analyzed using an 18-MHz linear transducer. In all cases, diagnosis was confirmed by preoperative skin biopsy.
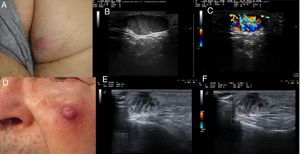
B-mode imaging showed well-defined, hypoechoic dermal and hypodermal lesions. Two of the tumors had homogeneous content. The other 3 were heterogeneous and contained hyperechoic septae in 1 case and hypoechoic lines running perpendicular to the skin surface in the other 2. The B-mode images also showed posterior acoustic enhancement in 3 of the tumors and thinning of the overlying epidermis in 2.
Most of the tumors had intense intralesional hypervascularity on color Doppler ultrasound. The vascular structures were predominant in the basal area of all tumors except the one in which hyperechoic lines had been observed by B-mode ultrasound. In this case, a strong Doppler signal was detected among the septae (Figs. 1 and 2).
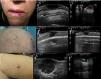
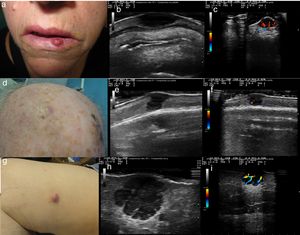
A (case 1), Firm, indurated, erythematous lesion with a diameter of 1cm on the left side of the lower lip; B, B-mode image showing a homogeneous hypoechoic dermal lesion with well-defined margins; C, Color Doppler image showing predominant hypervascularity in the basal area; D (case 2), Subcutaneous nodule with a diameter of 8mm located in the right parietal region and covered by pink skin; E, B-mode image showing a hypoechoic dermal/epidermal lesion; F, Color Doppler image showing a weak signal around the lesion; G (case 3), Pink nodule with a diameter of 18mm on the internal aspect of the right thigh; H, B-mode image showing a hypoechoic polylobular dermal/hypodermal lesion with well-defined margins, posterior acoustic reinforcement, and predominant intralesional vascularity in the septae.
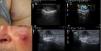
A (case 4), Subcutaneous lesion with a diameter of 20mm, an elastic consistency, and a violaceous surface on the right buttock; B, B-mode image showing a hypoechoic hypodermal lesion with heterogeneous content and hypoechoic plume-of-smoke lines running perpendicular to the epidermis, well-defined margins, and posterior acoustic reinforcement; C, Color Doppler image showing intralesional vascularity; D (case 5), Erythematous nodule over a subcutaneous lesion with a diameter of a 22mm on the left cheek; E, B-mode image showing a hypoechoic dermal lesion with heterogeneous content and hypoechoic plume-of-smoke lines running perpendicular to the epidermis; F, Color Doppler image showing predominant intralesional vascularity in the basal area.
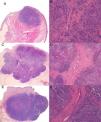
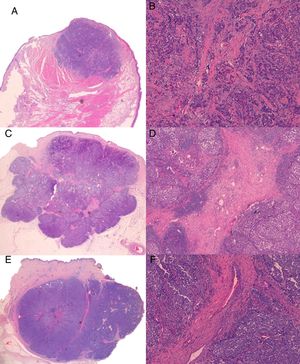
A full histologic examination was possible in 3 of the tumors; the other 2 (cases 2 and 5) regressed spontaneously after the skin biopsy (diagnosis was confirmed by histologic examination of the surgical specimen). Three of the tumors showed a solid growth pattern. One had spiculated margins and the other 2 had better defined margins and a lobular structure. All 3 tumors had fibrous trabeculae separating the cells; the first tumor had fine bands with scant vascular structures, while the other 2 had thick fibrous bands containing numerous vascular structures (Fig. 3). All 5 tumors were studied by immunohistochemistry and were positive for chromogranin, synaptophysin, and CD56. Paranuclear staining was observed for cytokeratin 20 and negative results were detected for CD45, S-100 protein, and thyroid transcription factor 1.
A, Case 1: Lesion with solid growth pattern and slightly spiculated margins (hematoxylin-eosin [H-E); B, Detail of fibrous trabeculae in the form of fine bands with scant vascular structures (H-E, original magnification ×100); C (case 3), Tumor with clearer margins and a lobular structure with fibrous bands that appear to correspond to the hyperechogenic intralesional lines observed by B-mode ultrasound (H-E); D, Detail of thick fibrous bands containing numerous vascular structures (H-E, original magnification ×40); E (case 4), Lobular lesion with a thick fibrous band perpendicular to the epidermis that appears to correspond to the hypoechoic line detected by B mode ultrasound; F, Detail of thick central fibrous band with wider vascular structures than in case 3 (H-E, original magnification ×100).
There are very few descriptions of the ultrasound features of MCC in the literature, as ultrasound is mainly used to study locoregional lymph nodes in this setting.4,5 Wortsman et al.6 described hypoechoic dermal and hypodermal lesions with ill-defined margins and intense hypervascularity. Although the ultrasound findings for the 5 tumors in our series were quite diverse, they all had similar characteristics to those described in the literature, except for the tumor margins, which were well-defined in all cases.
Other common ultrasound findings reported for MCC, and also observed in our series, are posterior acoustic reinforcement and thinning of the overlying epidermis.7–9 In a recent article, Hernández-Aragüés et al.7 described hypoechoic linear bands with a plume-of-smoke appearance perpendicular to the skin surface in 2 cases of primary MCC; Doppler imaging showed vascularity in these areas. Two of the tumors in our series had hypoechoic bands, but the vascular structures were located elsewhere.
Histologically, 2 of the tumors in our series (the one with the plume-of-smoke appearance on ultrasound and the one containing hyperechoic septae) had thick fibrous bands separating the cells. Although these fibrous trabeculae featuring abundant vascular structures appeared to correspond to the perpendicular hypoechoic lines and the hyperechoic septae detected by ultrasound, we found no histologic differences to explain the different echogenicity of these structures.
In conclusion, our analysis of the 5 cases in our series and those reported in the literature did not reveal a distinctive ultrasound pattern for MCC and we were also unable to determine the diagnostic and prognostic significance of some of the features observed. Notwithstanding, skin ultrasound is undoubtedly a useful imaging tool for determining tumor location, extension, vascularity, and relationship to adjacent structures. As such, it can aid in the planning of surgery. In addition, it provides an objective measure of tumor size and extent of deep invasion and can also identify subcutaneous or in-transit metastases, enabling better staging and providing preliminary prognostic information.10
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: García-Harana C, Fernandez-Canedo I, Rodriguez-Lobalzo S, de Troya-Martín M. Carcinoma de células de Merkel: ¿existe un patrón ecográfico distintivo?. Actas Dermosifiliogr. 2019;110:503–506.