Melanocytic pigmented lesions pose a real challenge for clinicians and pathologists. They are clinically and histologically very similar and it is essential to know the evolution of these lesions to establish a proper diagnosis.
An 87-year-old man came to the Dermatology Department for the evaluation of a tumor in the right shoulder. The lesion had developed over 20 years and had presented changes in the last three months (growth and hyperpigmentation). He did not have other accompanying symptoms.
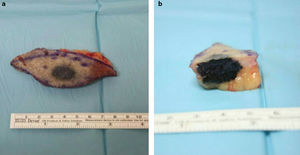
We observed a 1.7-cm indurated, pigmented nodule, which seemed embedded in the skin of the right shoulder with apparently no subcutaneous component. A simple excision adjusted to palpable limits of the lesion was performed (Fig. 1a). After surgery we observed a macroscopically intense pigmentation affecting the deep dermis (Fig. 1b), so we sent the sample to the Pathology Department with a suspected diagnosis of malignant melanoma.
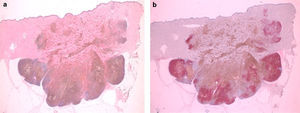
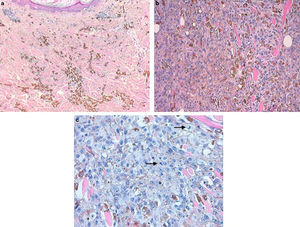
Histologically we observed one intensely pigmented lesion, localized in the dermis and subcutaneous tissue, with no intraepidermal component or connection to the epidermis, which was flattened and showed some hyperkeratosis. The lesion itself showed a pattern of expanding growth with various lobes (Fig. 2a). The central area consisted of numerous melanophages (Fig. 3a), while the periphery and deep lesion area were composed of melanocytic cells with an epithelioid or slightly fusocellular nature, moderate cytologic atypia and pleomorphism (Fig. 3b). Those cells were of medium and large size, with slightly eosinophilic cytoplasm and vesicular nuclei, most of them with a central nucleolus or smaller sized nucleoli. We counted up to four mitoses per HPF in the proliferation areas, some of them showing atypia (Fig. 3c). A scarce inflammatory component – consisting of lymphocytes interspersed with tumor cells – was observed. We did not observe any foci of necrosis or images of lymphovascular or perineural invasion. An immunohistochemistry analysis using red chromogen revealed that the melanocytic cells were only found in the deep and peripheral part of the lesion, while the rest of the cells corresponded entirely to melanophages (Fig. 2b). With these findings, the diagnosis of malignant blue nevus (MBN) was established.
We carried out an extension study on the patient, including a complete clinical examination to rule out the presence of another pigmented lesion that could be considered the primary tumor, but it turned out completely normal. We also performed blood tests with WBC, biochemistry and liver panels, and also a PET-CT with no altered results from average normal ranges: no systemic disease was evident. Twelve months after surgery, no signs suggesting recurrence or systemic disease were observed and the patient remains asymptomatic from all points of view.
Malignant blue nevus (MBN) was a term introduced by Allen and Spitz to describe lesions that are similar to blue nevi but can show a malignant behavior, even lethal.1 Currently, the term MBN is controversial and some authors like Ackerman2 do not recommend its use.
This diagnosis has been used in different clinicopathologic situations, such as melanomas that arise on a blue nevus, usually on the cellular ones. This probably could be our case if we consider the evolution of the lesion. MBN could also refer to new melanomas that contain elements that are reminiscent of a blue nevus. Critics of the term claim the supposed incongruity of referring to a malignant nevus, when the definition of nevus includes benignity. Therefore, a synonym for MBN that has been proposed today is blue-nevus-like melanoma. This seems to be the most appropriate way to refer to these lesions.
We can find several published series of MBN where the lesions had the same clinical outcomes as regular malignant melanomas, especially if we consider survival and recurrence. But some isolated studies suggest that the clinical course is usually more aggressive in the MBN cases.
The histological criteria for the diagnosis of MBN are not well defined, but almost every published paper agrees that these lesions show cytological atypia, a high mitotic index and the presence of atypical mitosis. They usually present necrosis and an infiltrative growth rate.1–3
Differential diagnosis of these lesions can be complicated. First, distinguishing between nodular and metastatic melanoma is needed. This is mandatory in order to determine if the lesion we are studying is a skin metastasis or a primary tumor. Some imaging tests may be helpful for this task, such as PET-CT. Another important differential diagnosis to consider is what is called “animal-type melanoma,” which has a low mitotic index and a slight melanophagic component.4 The third differential diagnosis must be made with pigmented epithelioid melanocytoma (PEM), which is very similar to the animal-type melanoma and also to the benign blue nevus. PEM is usually a “de novo” lesion, more frequent in young patients. It also shows a low mitotic index and melanophagic component. Finally, we should consider the differential diagnosis with the atypical cellular blue nevus, which is usually a well-demarcated lesion with intermediate atypia and a low mitotic index, but with no necrosis.5
Conflict of interestsThe authors declare no conflict of interests.