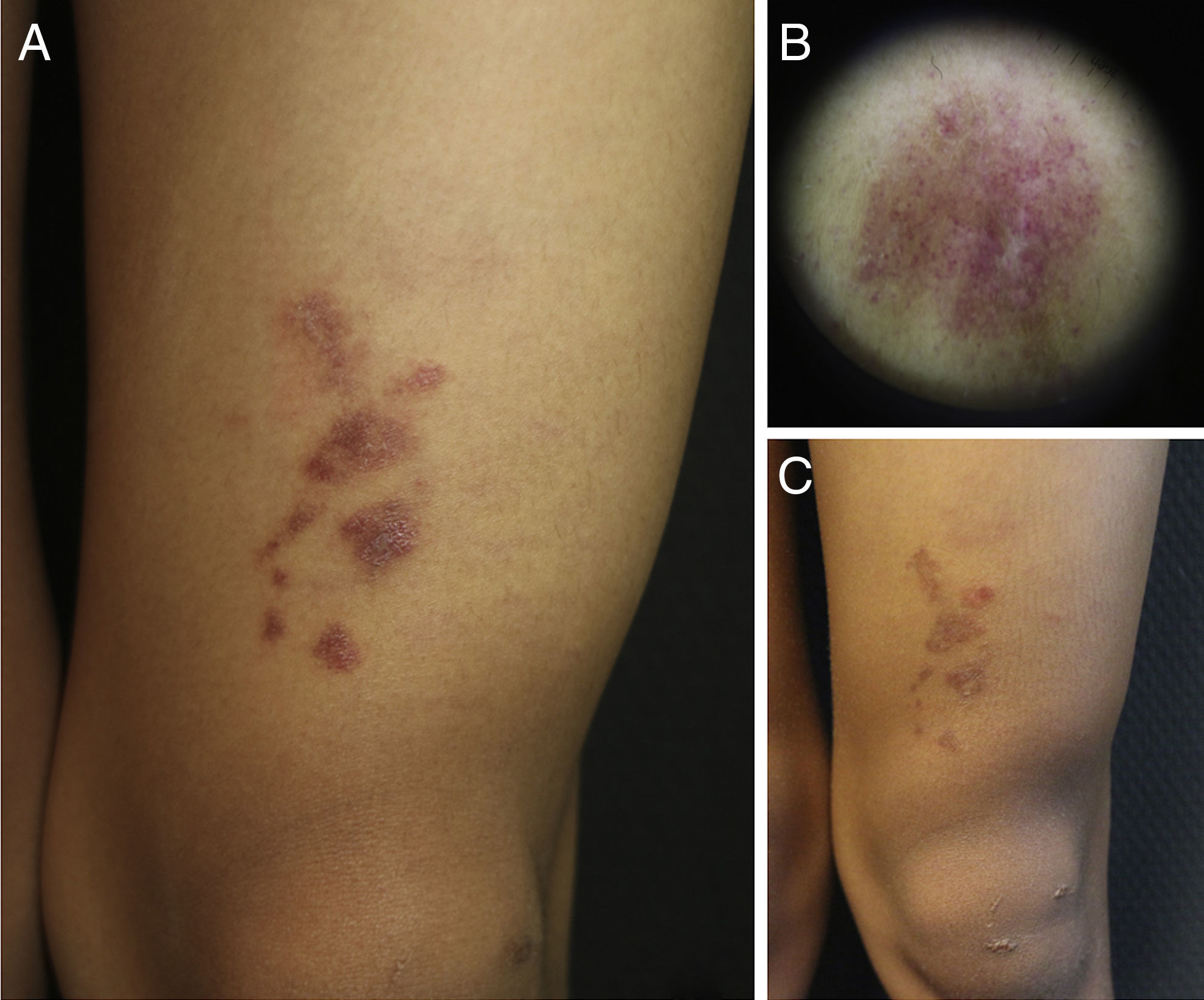
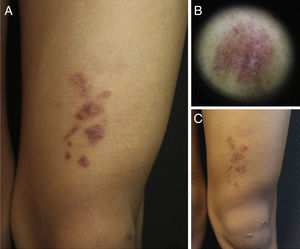
A 9-year-old boy, with Fitzpatrick skin phototype V, was seen for an asymptomatic lesion on his left knee, diagnosed at birth by the neonatologist as a café au lait spot. The lesion was described in the neonatal discharge report as a brownish macule measuring 1.5×1.5cm, on the left knee, observed during the first examination of the newborn infant. However, there had been no subsequent follow-up and the child had not been seen in dermatology before his visit to our outpatient clinic. On examination of the child's left knee, a number of shiny erythematous-purpuric plaques were observed in a linear distribution, with a slightly pigmented halo and fine superficial desquamation. Dermoscopy revealed lobules and pink dots on a diffuse coppery-brown background, and a fine brown network (Fig. 1). With a diagnostic suspicion of a lichenoid rash, lichen aureus (LA), or nevus flammeus—because of its congenital nature—one of the plaques was biopsied.
Clinical and dermoscopic images of the patient. A, Grouped, shiny erythematous-purpuric plaques with fine superficial desquamation on the left knee. B, Dermoscopy showing red dots on a brownish-gray background with a fine network. C, Clinical course of the lesion after several attempted treatments. Note persistence of the plaques but with a slight reduction in color.
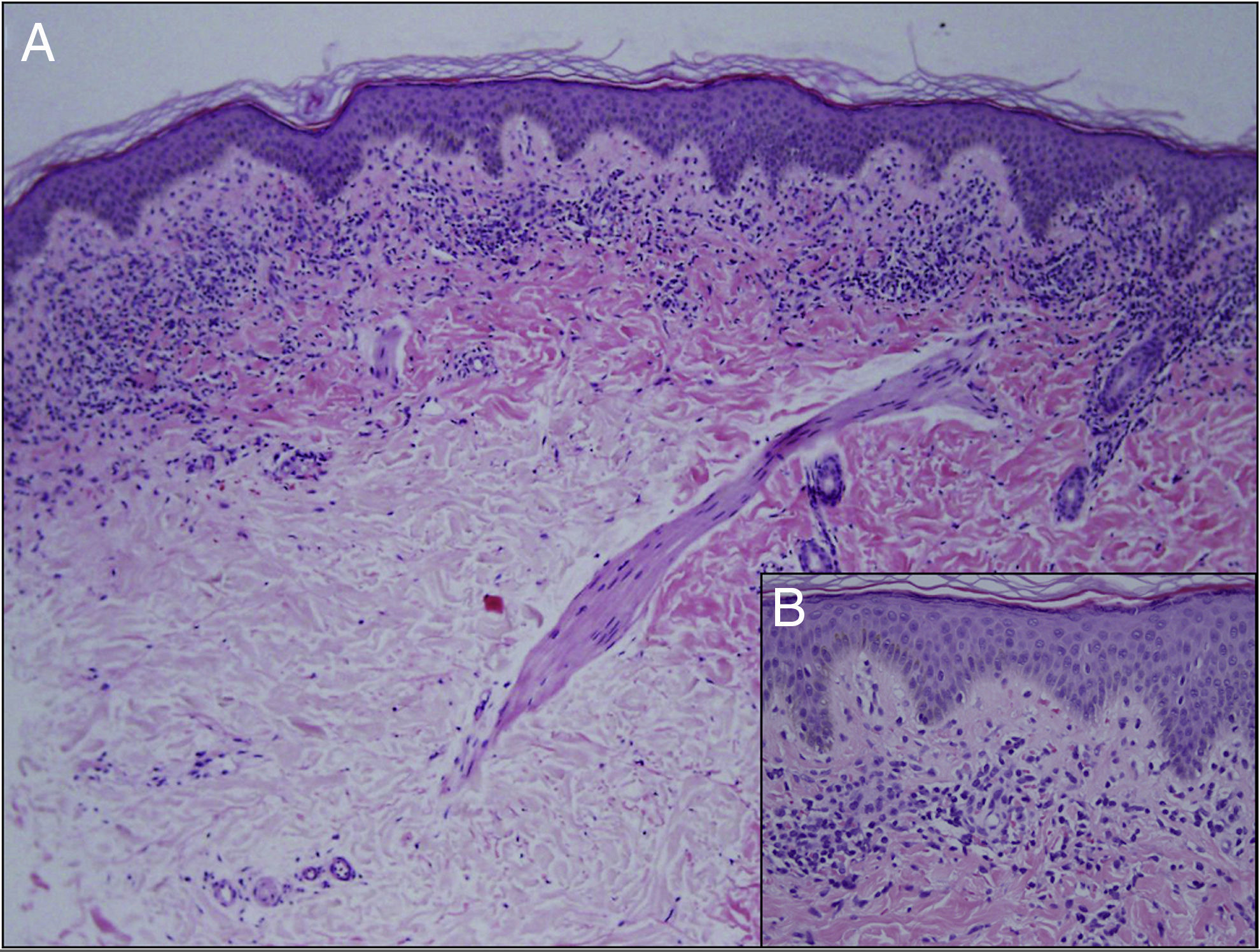
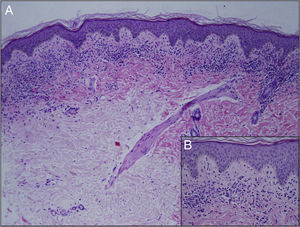
Minimal hyperkeratosis, mild acanthosis, and mild epidermal spongiosis were observed on histology, together with a dense, band-like lichenoid inflammatory infiltrate in the papillary dermis, with occasional apoptotic keratinocytes and extensive red cell extravasation (Fig. 2). The patient was diagnosed with LA and treatment was started with 0.1% methylprednisolone aceponate for a month. No improvement was observed at the follow-up visit at 4 months, although his parents considered the lesion to be less infiltrated. He was then prescribed a 1-month course of treatment with 0.25% prednicarbate cream. Four months later the lesion remained unchanged. Given the benign and asymptomatic nature of the lesion and the lack of improvement, it was decided to abstain from further therapy and to keep the patient on follow-up. At the time of writing, the lesion continues unchanged.
LA is a rare entity in children, with a higher incidence in young adults.1 It was first described in 1959 by Martin, though it was not until 1960 that Calman coined the current name based on its characteristic golden halo.2 It is included in the group of pigmented purpuric dermatoses.
The etiology of LA is unknown, although several etiological factors and triggers have been proposed, such as infection, trauma, toxins, venous insufficiency, contact allergy, and underlying autoimmune diseases.2,3 LA usually presents as brownish, violaceous, or copper-colored macules and papules or plaques, with a peripheral golden-yellow halo, fine superficial desquamation, and shiny surface.1,3,4 Lesions are typically unilateral on the limbs or trunk, although they can be bilateral in around 10% of cases.1 Up to now, LA has most often been described as a lesion affecting the lower limbs, but a recent series of 25 patients found the same prevalence on arms and legs.1
Dermoscopy is a useful diagnostic tool in this entity. The reported findings are summarized in 4 points: a diffuse brownish or copper-red background, red lobules or dots, grayish dots, and a pigmented network or pseudonetwork.5
Histologically, there is a characteristic band-like lymphocytic infiltrate and red-cell extravasation with hemosiderin deposits.6 Hemosiderin may not be seen in the early stages on staining with hematoxylin and eosin; Perls stain is useful in these cases.1,7 The presence of a periadnexal and perineural infiltrate is a histological finding disputed in the literature and is more characteristic of lichen striatus. In their series of 25 patients, Zeng YP et al.1 found a periadnexal infiltrate in 12 patients and a perineural infiltrate in 5, more common than previously reported in LA.
It should be noted that LA can occasionally be confused with mycosis fungoides (MF), and some authors even defend a relationship between the 2 diseases, suggesting that LA may have the capacity to progress to MF.7
The diagnosis is clinical and pathological and the differential diagnosis should include other pigmented purpuric dermatoses, mycosis fungoides, lichen striatus, blaschkitis, traumatic contusions, contact dermatitis, and Langerhans histiocytosis.1,8
The prognosis of the disease is variable and unpredictable; most commonly there is spontaneous resolution with subsequent recurrences.3,4,9 A number of therapeutic alternatives have been proposed, including topical corticosteroids, phototherapy, and calcineurin inhibitors, although there is still no truly effective option.4
We have presented a case of segmental LA in a patient with a high skin phototype, a factor that altered the color of the lesion, giving it a more pigmented appearance than usual and masking the characteristic golden-yellow halo. Dermoscopy and histology enabled us to reach a correct diagnosis. Neonatal LA has been reported in the literature,10 but we have found no other cases of congenital LA. In our patient, the lesion observed when he was 9 years old was at the same site as the lesion described in the first neonatal examination. In addition, the patient's parents described it as the same lesion. This would all support its congenital nature, though this cannot be confirmed, as no dermatologic follow-up was performed on this patient after birth.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Corral-Magaña O, Escalas J, Bauzá A, Martin-Santiago A. Liquen aureus: ¿un caso congénito?. Actas Dermosifiliogr. 2017;108:965–966.