Hair shaft disorders, involving dysplastic abnormalities in the shaft, may be either congenital or acquired. Two large categories have been defined according to the presence or not of hair fragility. A diagnosis can usually be made after taking a thorough medical history and performing a physical examination. Trichoscopy has become a useful, cost-effective tool in recent years, particularly for examining the hair of children, because it facilitates inspection without removal of hairs. Structural abnormalities in the hair shaft are sometimes clues to the diagnosis of more complex diseases in which early treatment can improve prognosis. This review describes key features that enable the diagnosis of the most common hair shaft disorders and discusses the various treatments currently available.
Las displasias pilosas corresponden a alteraciones en la estructura del tallo del cabello y pueden ser congénitas o adquiridas. Se clasifican en dos grandes grupos según la presencia o no de fragilidad capilar. En la mayoría de los casos la valoración del paciente, la anamnesis y la exploración física nos llevarán al diagnóstico. En los últimos años, el uso de la tricoscopia se ha posicionado como una técnica útil y coste efectiva, sobre todo en niños, ya que permite lograr una adecuada exploración sin tener que arrancar los cabellos.
En algunas ocasiones las alteraciones en la estructura del cabello serán la clave diagnóstica de enfermedades más complejas, en las que la instauración de un tratamiento precoz puede mejorar el pronóstico.
El propósito de esta revisión es aportar las claves que permitan diagnosticar las displasias pilosas más frecuentes y valorar las alternativas terapéuticas disponibles en la actualidad.
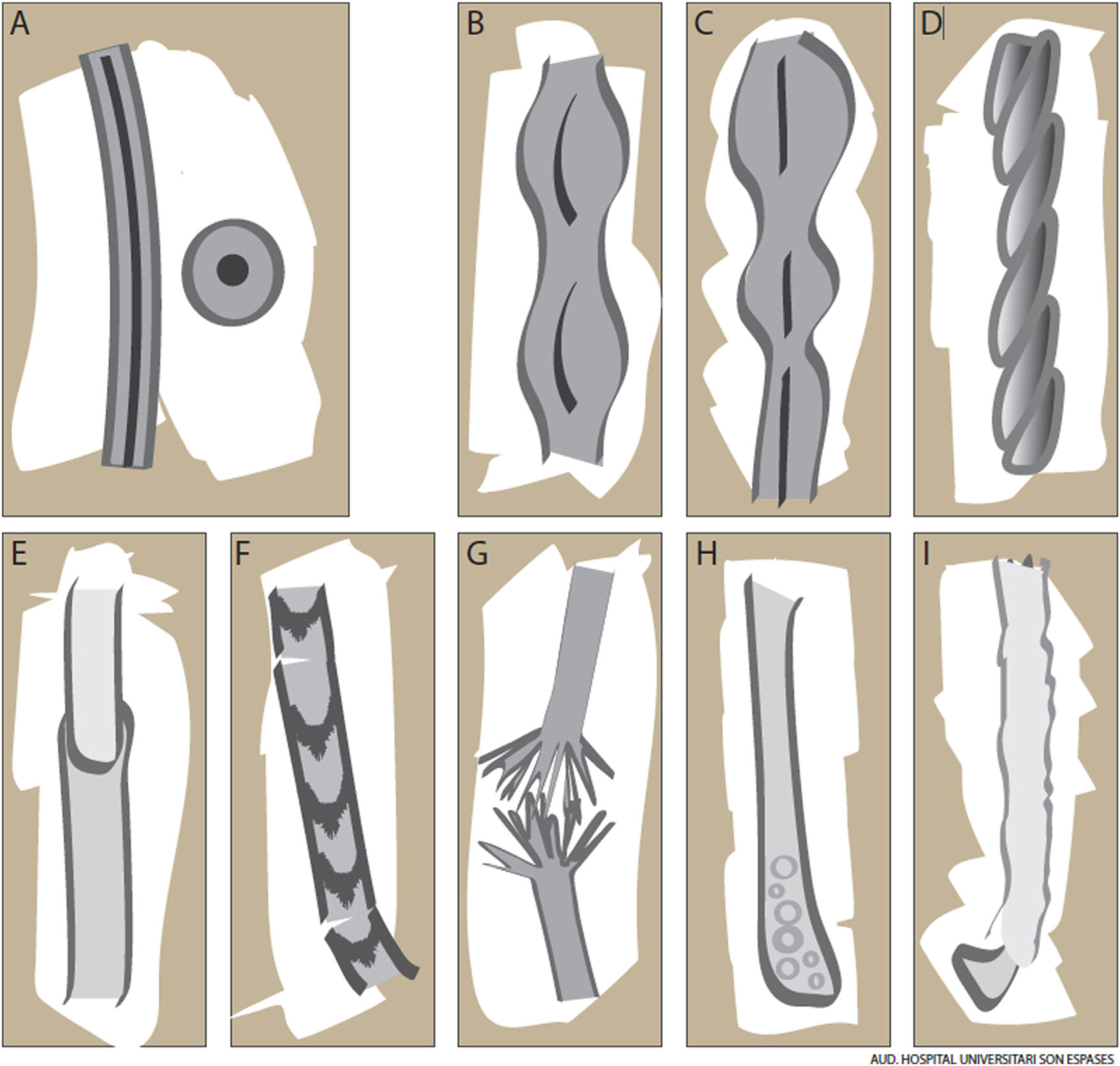
The hair shaft is composed of a cortex, a protective cuticle, and, in the case of terminal hairs, an inner medulla (Fig. 1A). This structure confers unique properties in terms of strength, flexibility, and resistance to the environment.1
Hair shaft disorders are induced by environmental factors or genetic mutations.2,3 Patients, or their parents, typically consult about changes to appearance or texture, increased fragility, and poor growth.1 This review describes key features for recognizing the most common hair shaft disorders and evaluates triggers, prognosis, and current treatments.
Targeted history taking is important when dealing with hair complaints. Examples of questions are “When did the problem begin?” “Was it present at birth or did it begin later?” “Have you noticed any changes to teeth or nails?” “Do any of your relatives have similar findings?” and “How do you look after your hair/What cosmetic products do you use?” It is also important to ask about frequency of washing, styling techniques, and use of hair gels, permanent straighteners, and hair dryers or curling tongs.1,4
When examining the hair it is important to1:
- •
Assess its overall appearance, including luster, curl, and color.
- •
Determine whether the abnormality is focal or diffuse.
- •
Perform a pull test (Sabouraud sign), which consists of grasping tufts of 20 to 60 hairs between the index finger and thumb and gently pulling on these in different areas of the scalp. The test is positive when more than 10% of the hairs come out.
- •
Perform a tug test, which consists of holding a tuft of hair between the fingers several centimeters from the root and tugging it to detect the presence of fragile areas.
- •
Examine the scalp.
- •
Use noninvasive tests such as photography and trichoscopy to complement the physical findings.
- •
In selected cases, use semi-invasive tests such as trichogram analysis, optical microscopy, and electron microscopy.5
- •
Determine the need for a scalp biopsy where appropriate.
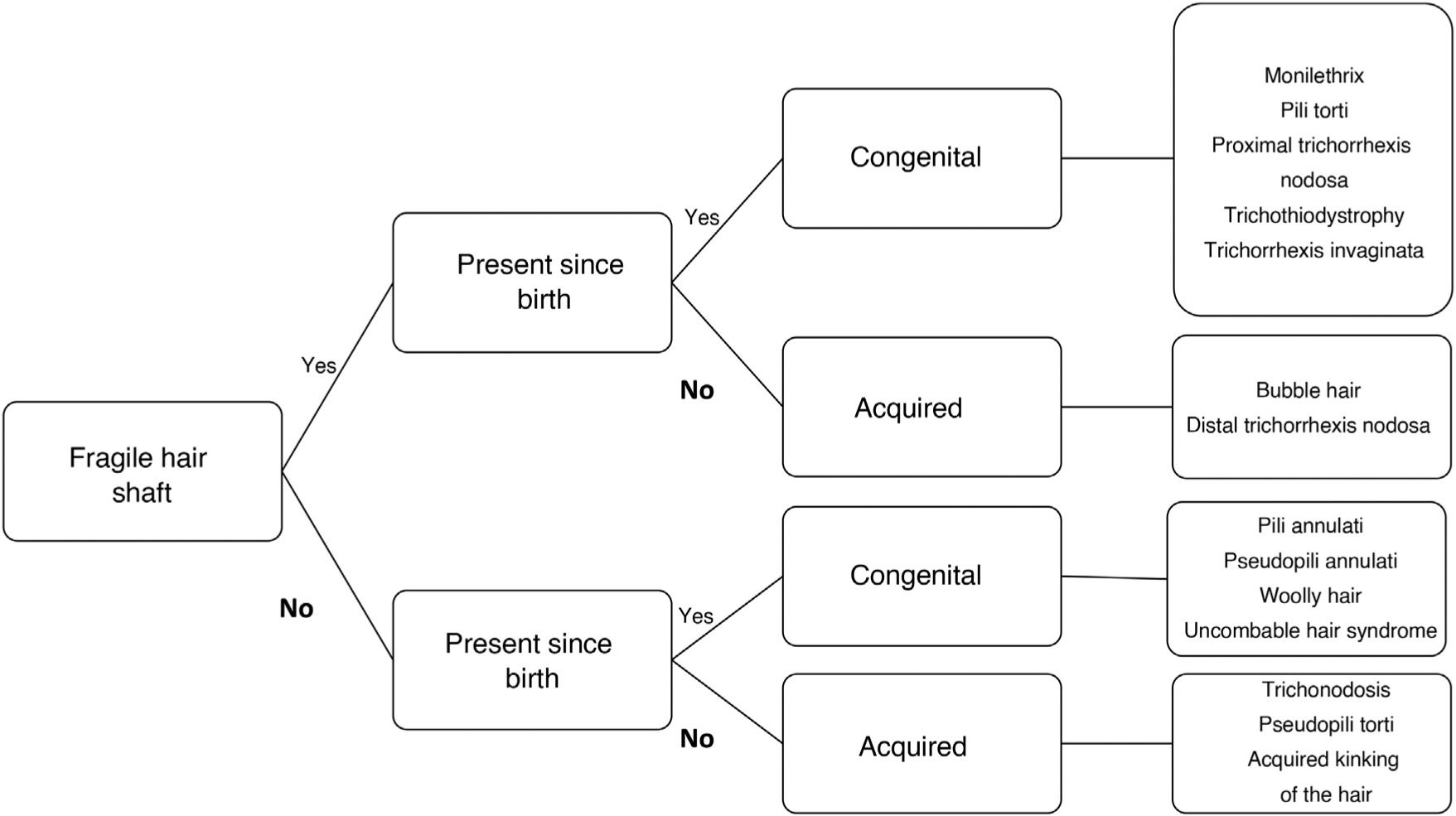
Hair shaft disorders can be difficult to classify because of overlapping clinical findings. One way of classifying them is according to the presence or absence of hair shaft fragility (Fig. 2).6 A thorough history combined with meticulous examination of the hair and additional techniques such as trichoscopy and optical or electron microscopy should result in a correct diagnosis (Table 1).
Steps in the diagnosis of hair shaft disorders.
Adapted from Mirmirani et al.1
Classification of Hair Shaft Disorders.
| Hair shaft disorders with hair fragility |
| Monilethrix |
| Pseudomonilethrix |
| Pili torti |
| Menkes syndrome (kinky hair) |
| Netherton syndrome (trichorrhexis invaginata) |
| Trichothiodystrophy |
| Trichorrhexis nodosa |
| Bubble hair |
| Loose anagen hair syndrome |
| Hair shaft disorders with minimal or no hair fragility |
| Trichonodosis |
| Pili annulati |
| Pseudopili annulati |
| Woolly hair |
| Diffuse partial woolly hair |
| Woolly hair nevus |
| Acquired progressive kinking of the hair |
| Acquired partial kinking of the hair |
| Straight hair nevus |
| Uncombable hair syndrome (pili canaliculi et trianguli) |
Adapted from Ferrando et al.2
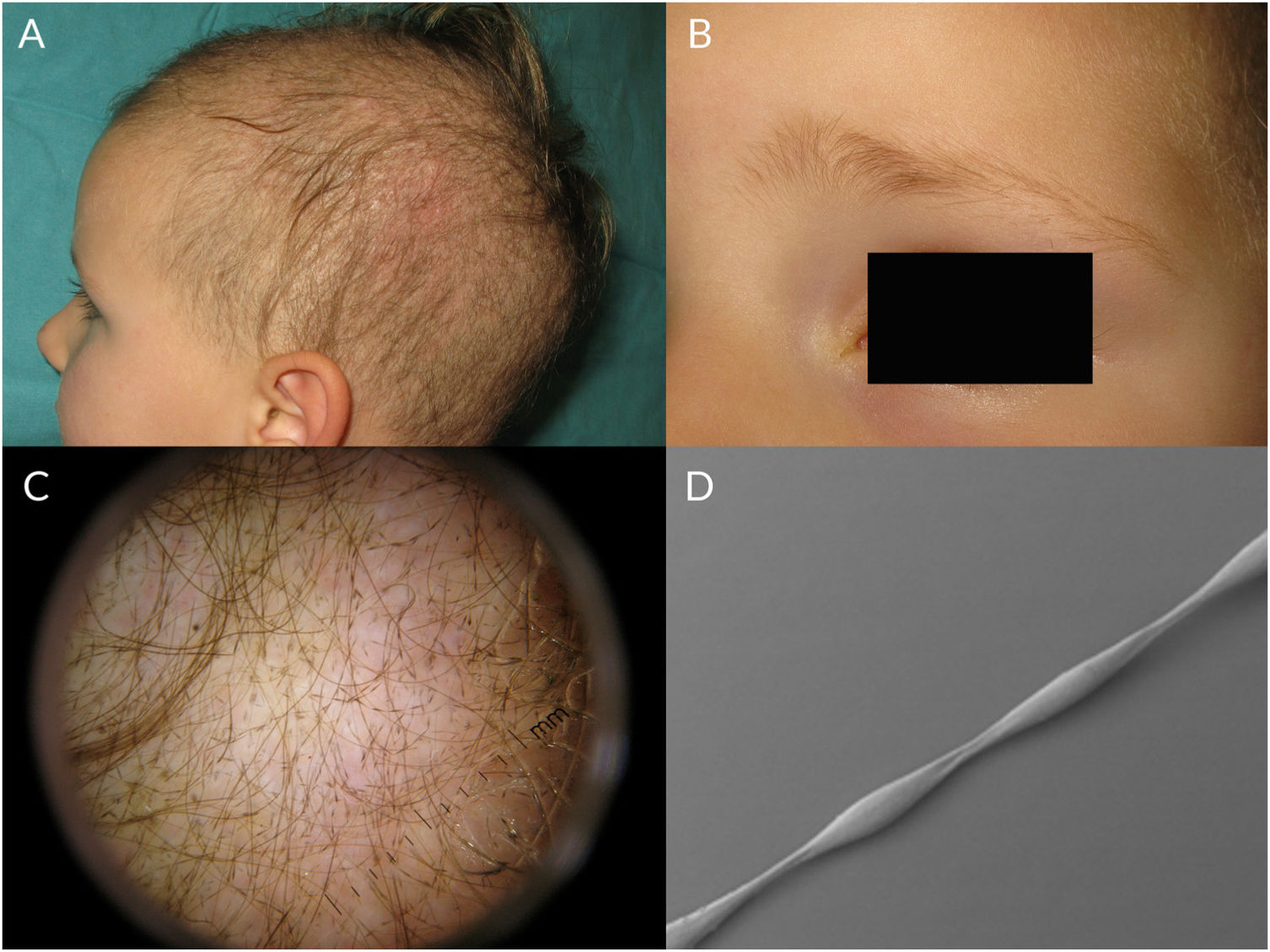
Monilethrix is characterized by regularly spaced hair shaft constrictions (Fig. 1B). Clinically, the hair tends to be normal at birth, but after a few months it becomes dull, short, and fragile; this results in hypotrichosis, which predominantly affects the occipital region of the scalp, which is more prone to friction (Fig. 3A). Patients with monilethrix usually have pronounced follicular hyperkeratosis on the scalp. This can be focal (with predominant occipital involvement) or diffuse. Marked follicular hyperkeratosis may also be seen in patients with keratosis follicularis spinulosa decalvans (Siemens syndrome).2 Monilethrix can also affect the eyebrows (Fig. 3B), eyelashes, and body hair. Patients may also show nail changes such as koilonychia.7–10
Monilethrix is inherited in an autosomal dominant pattern, with high penetrance and variable expressivity. Mutations in the KRT81, KRT83, and KRT86 genes, which code for the keratins Hb1, Hb3, and Hb6, cause abnormal keratinization of the hair shaft cortex.7,8,11,12 Monilethrix also has an autosomal recessive form caused by mutations in DSG4, which codes for desmoglein 4. These mutations result in alterations to plakoglobin and desmosomes in the hair shaft.8 Monilethrix has also been linked to keratosis pilaris, hereditary koilonychia, and Holt-Oram syndrome.7
Trichoscopic examination shows clear narrowing of the hair shaft (Fig. 3C). Other possible findings include erythema, perifollicular hyperkeratosis, and even follicular papules. The hair shafts have a beaded appearance formed by nodes, which correspond to areas with a normal diameter, and regularly spaced, unmedullated constrictions (internodes) (Fig. 3D), which is where breakage tends to occur.7,13–16
The clinical course of monilethrix is variable. In most cases, the condition lasts for life, although partial and complete remissions have been reported in the summer and in association with pregnancy, oral contraceptive use, and older age.17 Variable responses have been described for treatment with topical and oral retinoids, topical minoxidil 2% and 5%, oral minoxidil, and acetylcysteine. There have been reports of recurrence on discontinuation of oral retinoids.18–20
PseudomonilethrixPseudomonilethrix presents with areas of diffuse or focal hair loss. It is characterized by short, beaded hairs that break once they have reached a few millimeters in length.2 It occurs at older ages than monilethrix. Unlike monilethrix, it does not show follicular hyperkeratosis and the hair shaft presents irregularly spaced swellings rather than constrictions. In this case, the internodes have the diameter of a normal hair shaft (Fig. 1C). Microscopic examination shows flat, indented swellings.21
Camacho et al.21 proposed the following classification system for pseudomonilethrix:
Bentley-Phillips Familial Pseudomonilethrix (Type I)Bentley-Phillips familial pseudomonilethrix is inherited as an autosomal dominant disorder with variable penetrance; it was described by Bentley-Phillips and Bayles in 1973.22 It only affects hair on the scalp and can give rise to longitudinal fractures. Onset is prepubertal.
Acquired Pseudomonilethrix in Hair Shaft Disorders (Type II)Acquired pseudomonilethrix is observed in certain hair shaft disorders with increased hair shaft fragility, such as monilethrix, pili torti, trichorrhexis nodosa, woolly hair, bubble hair, acquired kinking, and acrodermatitis enteropathica.23 It can manifest as androgenetic alopecia or trichotillomania. Styling and brushing can aggravate the condition.
Iatrogenic Pseudomonilethrix (Type III)Iatrogenic pseudomonilethrix is seen in hair shaft disorders characterized by hair fragility and individuals with fine, light-colored hair; it is caused by trauma to the hair during the preparation of trichograms (eg, compression by Pean forceps while traction is applied or pressing of the hairs between glass slides).24,25
As iatrogenic pseudomonilethrix is a laboratory artifact it does not need treatment.21 The use of ultrasound gel as an immersion fluid can also give the impression of flattened hair shafts on trichoscopy. A similar effect is produced by hair gel and patients should therefore be advised not to use products of this type before trichoscopy.14
Pili TortiPili torti are flattened hair shafts twisted at regular or irregular intervals around their axis at angles of 90°, 180°, or even 360° (Fig. 1D).16 It has been suggested that cell vacuolation and irregularities in the thickness of the outer root sheath might affect the inner sheath, causing the hair to twist around its axis.21 Pili torti can affect the head and other hair-bearing areas of the body, including eyebrows, eyelashes, underarm hair, and pubic hair. Hair becomes more fragile, leading to breakages and focal or diffuse hypotrichosis. Pili torti tends to cause longitudinal splitting (trichoptilosis). Physical examination shows short dry hair that acquires a sequin-like appearance upon light reflection as the angles in the hair mean that only some stretches reflect the light.21
Congenital pili torti has been linked to mutations in BSC1L, ST14, and CDH3. Pili torti can be inherited in an autosomal dominant or recessive pattern and can appear in isolation or in association with other diseases, such as type 2 congenital pachyonychia and Björnstad syndrome, Beare syndrome, Bazex syndrome, Menkes syndrome, and Crandall syndrome.2,17 It can also occur in isolation in people with fine, blond hair.21 It sometimes improves after puberty, but can last for life.
Acquired pili torti is rare and has been linked to nutritional deficiencies, the use of certain drugs such as oral retinoids and erlotinib, and graft-versus-host disease.26,27 Ferrari et al.28 recently observed pili torti in the trichoscopic examination of a patient with frontal fibrosing alopecia and suggested that it might be a sign of fibrosis and a predictor of poor response to intralesional corticosteroids.
There are currently no effective treatments for pili torti. Physical and chemical trauma should be avoided.
Menkes Syndrome (Kinky Hair)Menkes syndrome is a rare neurodegenerative disease caused by abnormal copper metabolism.29 It was described by the pediatric neurologist John Menkes in 1962.30 It is an X-linked recessive disorder with an estimated incidence of 1 case per 300 000 live births in Europe.29 The causative gene, ATP7A, is located on chromosome X (Xq21.1), 4,9 (9q31-q32), 14, and 18 (18.26.0 cM) and encodes a transmembrane protein (ATP7A) that controls copper transport. Defective intestinal absorption of copper results in decreased levels of copper and ceruloplasmin in plasma and organs such as the brain, liver, bones, hair, and skin.6 Other pathogenic variants cause milder manifestations such as occipital horn syndrome and motor neuropathy.29
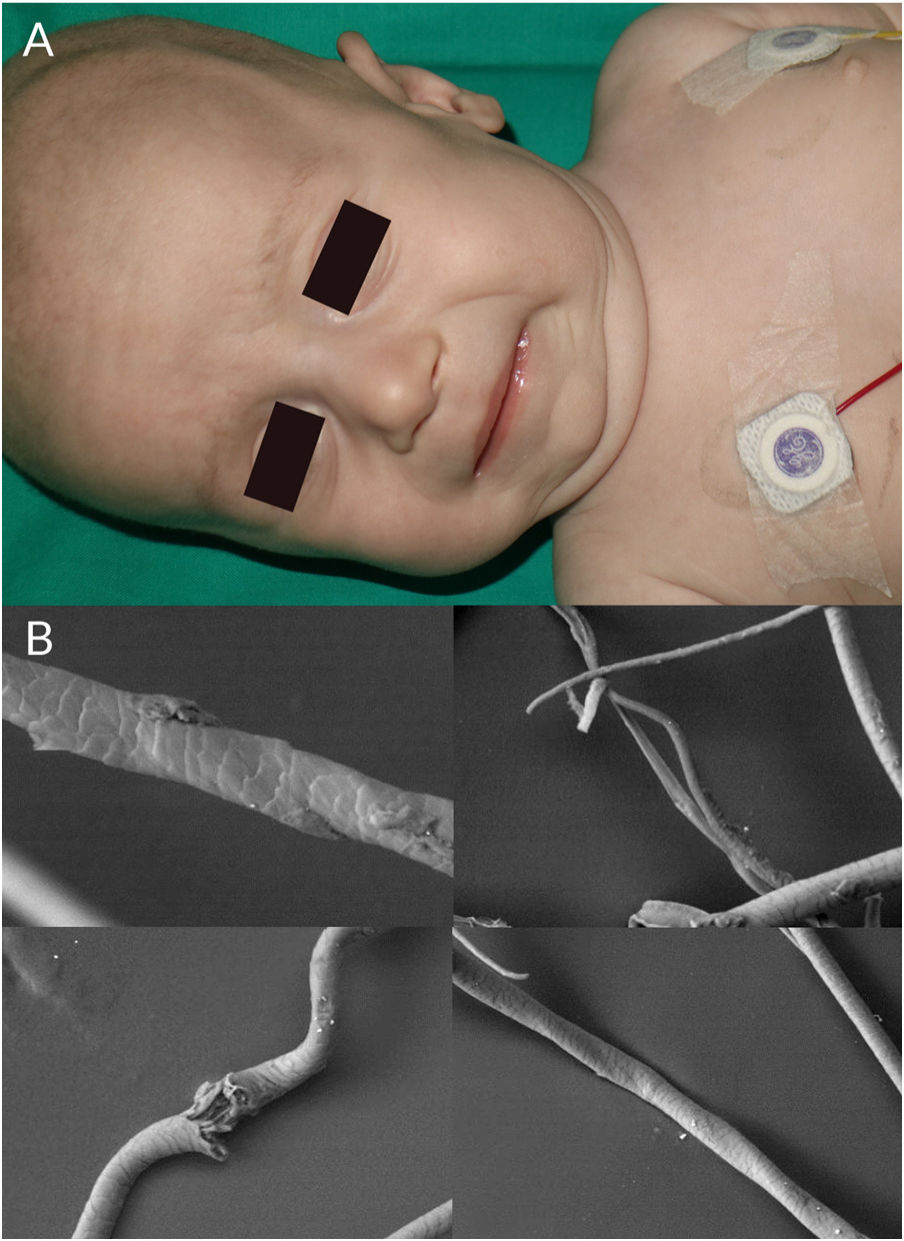
Children with Menkes syndrome typically show normal development up to 2 to 3 months of age, but then progressively show low weight, delayed psychomotor development, hypothermia, hypotonia, and seizures. Typical findings are pudgy cheeks, a pronounced Cupid's bow on the upper lip, and horizontal eyebrows, conferring a “partridge-like” facies (Fig. 4A). Additional findings are brittle hair, lax skin, joint hypermobility, inguinal hernias, and bladder and urethral diverticula. Milder forms of Menkes syndrome exist but their diagnosis can be delayed as they show very few neurological manifestations. There have been reports of Menkes syndrome presenting with subdural hematomas, cervical spine defects, rib fractures, and irregularities in the metaphysis of long bones; these manifestations were initially confused with signs of child abuse.31
Trichoscopic examination shows variations in hair shaft diameter, flattening and irregular twists (atypical pili torti), monilethrix, and/or trichorrhexis nodosa.2 These findings are observed in greater detail by optical or electron microscopy (Fig. 4B).
The diagnosis of Menkes syndrome is based on clinical findings and measurement of serum copper and ceruloplasmin levels.29
The natural disease course involves progressive loss of neurological functions, causing death in the first years of life.29 Early diagnosis is critical, as administration of subcutaneous copper-histidine can improve neurological prognosis.2,29
Netherton Syndrome (Trichorrhexis Invaginata)Netherton syndrome is a congenital autosomal recessive keratinization disorder of the skin, hair, and immune system.32 It was described by Comel (1949) and Netherton (1958). It is characterized by a triad of ichthyosis (circumflex linear ichthyosis or other types of congenital ichthyosis), hair shaft alterations, and signs of atopy (elevated immunoglobulin E, hypereosinophilia, eczema, rhinitis, food allergies, and asthma).33,34 Recurrent bacterial infections are common. Other findings include low weight, enteropathy, kidney failure, and intellectual disability.34 The estimated incidence of Netherton syndrome is 1 case per 100 000 to 200 000 live births.32 Mutations in the SPINK5 gene (chromosome 5q32) cause alterations in the LEKTI protein, leading to overexpression of 3 types of kallikrein: KLK5, KLK7, and KLK14.6,34
Patients with Netherton syndrome have short, lusterless, brittle hair. Trichorrhexis invaginata, or bamboo hair (hair with areas of invagination), is considered a specific finding (Fig. 1E).35 Low-magnification trichoscopy shows multiple, irregularly spaced, swellings along the hair shaft. Higher-magnification images show invagination of the distal hair shaft into the proximal part, forming a pathognomonic “ball in cup” appearance. When the hair breaks at the distal end, the proximal part acquires a golf-tee appearance. According to Rudnicka et al.,14,16 these trichoscopic findings are more easily observed in eyebrows, as these have a 10-fold higher density of bamboo hair than the scalp. Pili torti and trichorrhexis nodosa may also be present, but they are not characteristic and can improve with time and even resolve completely.36
A recent case-control study of 8 patients with Netherton syndrome reported a band-like pattern under polarized light microscopy that was more common than the pathognomonic invaginata trichorrhexis.33
There are no specific treatments for Netherton syndrome; prognosis is poor and mortality is high during the first years of life due to water loss, hypernatremic dehydration, and bacterial infections.32,34 Treatments are aimed at improving skin manifestations and include oral corticosteroids, retinoids, phototherapy, topical calcineurin inhibitors, and lactate lotions.17 New targeted therapies such as dupilumab that are being used in atopic dermatitis might be useful in Netherton syndrome, but more evidence is needed before they can be recommended.34,37
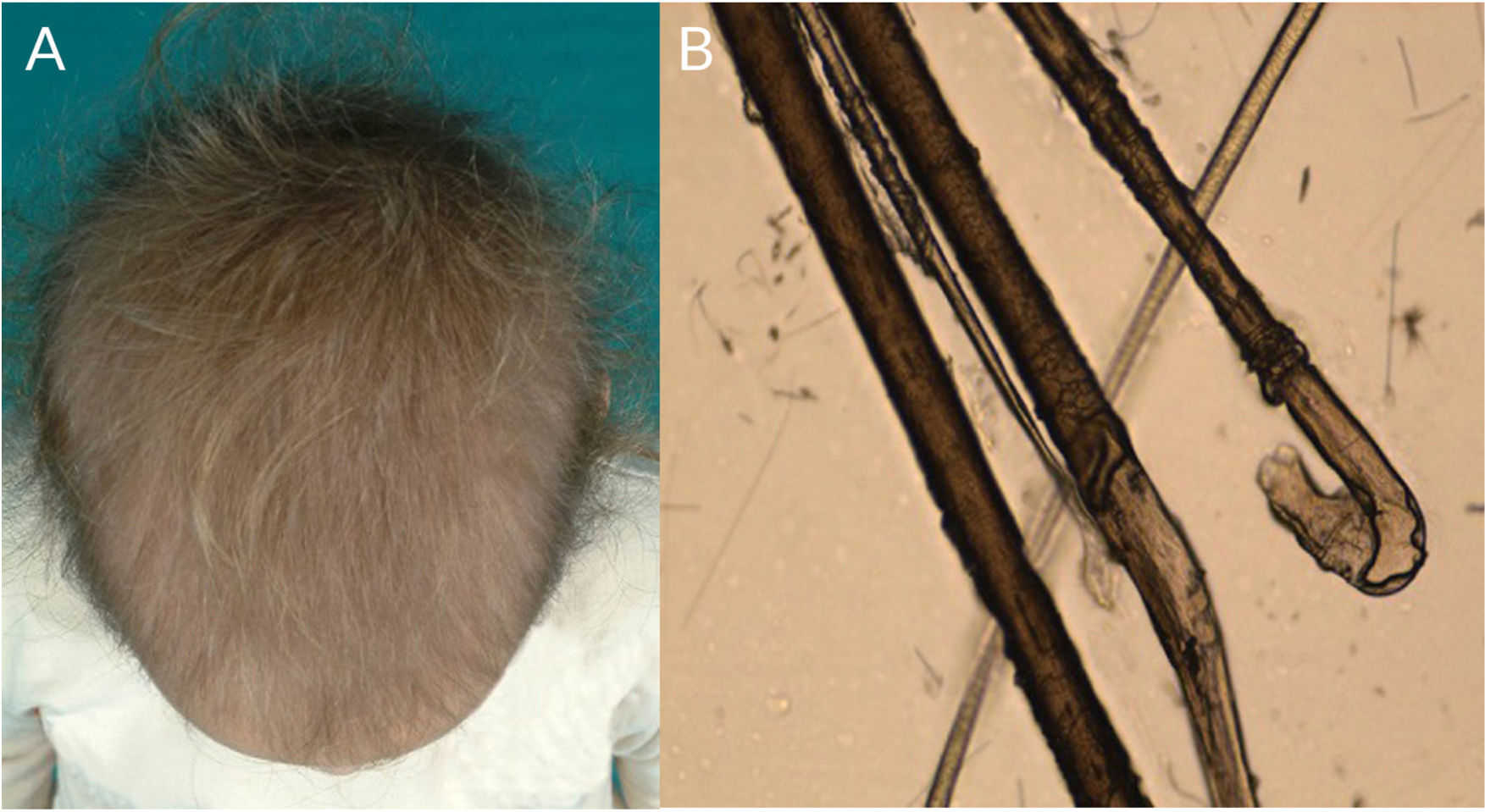
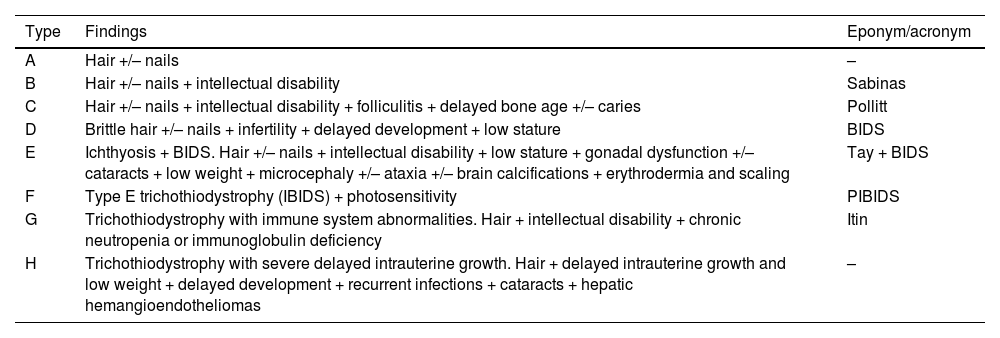
TrichothiodystrophyTrichothiodystrophy (TTD) comprises a heterogeneous group of diseases (Table 2).2 The main findings are hair abnormalities, microdolicocephaly, delayed psychomotor development, ichthyosis, signs of early aging, and photosensitivity.2,38 Nail abnormalities such as onychodystrophy, koilonychia, furrows, onychogryphosis, onychoschizia, yellowish discoloration, and unguis inflexus (longitudinal curvature of nails) are common.39 Trichothiodystrophy is inherited in an autosomal recessive pattern. Four genes have been described: XPD, XPB, p8/TTDA, and TTDN1. The first 3 have a role in DNA nucleotide transcription, repair, and cleavage. Unlike xeroderma pigmentosum, however, trichothiodystrophy is not associated with a greater predisposition to cancer.38–40 Clinical examination shows lusterless, fragile hair, hypotrichosis, and alopecia. These findings are also seen in the eyebrows and eyelashes. Trichothiodystrophy should be considered in the differential diagnosis when assessing a patient with congenital alopecia.39
Subtypes of Trichothiodystrophy.
| Type | Findings | Eponym/acronym |
|---|---|---|
| A | Hair +/– nails | – |
| B | Hair +/– nails + intellectual disability | Sabinas |
| C | Hair +/– nails + intellectual disability + folliculitis + delayed bone age +/– caries | Pollitt |
| D | Brittle hair +/– nails + infertility + delayed development + low stature | BIDS |
| E | Ichthyosis + BIDS. Hair +/– nails + intellectual disability + low stature + gonadal dysfunction +/– cataracts + low weight + microcephaly +/– ataxia +/– brain calcifications + erythrodermia and scaling | Tay + BIDS |
| F | Type E trichothiodystrophy (IBIDS) + photosensitivity | PIBIDS |
| G | Trichothiodystrophy with immune system abnormalities. Hair + intellectual disability + chronic neutropenia or immunoglobulin deficiency | Itin |
| H | Trichothiodystrophy with severe delayed intrauterine growth. Hair + delayed intrauterine growth and low weight + delayed development + recurrent infections + cataracts + hepatic hemangioendotheliomas | – |
Adapted from Itin et al.39.
Hair shaft alterations must be present for a diagnosis of trichothiodystrophy to be established. Samples must be taken from different areas of the scalp and examined by optical or electron microscopy.39 Findings include transverse fractures (trichoschisis), irregular hair shaft diameters and surfaces, and other features similar to those observed in trichorrhexis nodosa or pili torti. Polarized light microscopy shows a characteristic tiger tail appearance consisting of alternating light and dark bands (Fig. 1F) (Fig. 5).38 More recently described trichoscopic findings include glomerular hairs, which are kinked hairs with breakages at the level of the infundibulum.41 The tiger tail sign can also be visualized without the need for microscopic examination using polarized transilluminating dermoscopy with two dermoscopes and a mobile telephone or a dermoscope and a mirror.42
There are no specific treatments for the hair alterations associated with trichothiodystrophy, although avoidance of local trauma and administration of minoxidil 2% or oral cystine supplements have shown beneficial effects. Oral biotin has not proven effective in this setting.39
Trichorrhexis NodosaTrichorrhexis nodosa is a defect in which nodes along the hair shaft create weak points that result in easily broken hair. It is clinically characterized by fine, sparse hair. Trichoscopy with fluid immersion shows thickened nodes—sometimes accompanied by signs of breakage—along the shaft. Trichoscopy without fluid immersion shows small whitish areas that correspond to the breakage areas. Higher magnification will reveal numerous short fibers within the node, giving the appearance of the ends of two brushes facing each other (Fig. 1G).8,14 Sharp angles may also be seen.14 These findings can be visualized in greater detail by optical or electron microscopy.16
There are 2 types of trichorrhexis nodosa.
Distal Trichorrhexis NodosaDistal trichorrhexis nodosa is an acquired condition caused by exposure to external physical and chemical agents. It affects individuals with long hair and involvement is diffuse. It is more common in young women and is associated with excessive brushing and cosmetic treatments. Clinical findings include small whitish nodes at the distal end of the hair shafts, which tend to fracture longitudinally, causing split ends or trichoptilosis.2
People with acquired trichorrhexis nodosa should be advised to avoid excessive brushing, heat, wind, salt, and other chemical products and to use mild shampoos and conditioner.43
Proximal Trichorrhexis NodosaProximal trichorrhexis nodosa is less common than the distal form. The clinical findings are the same, but it is considered to be a more complex condition, as hair shafts can feature several nodes and it is often associated with hypotrichosis and alopecia. It is more common in Black people, who usually show hypotrichosis on the scalp and at other sites. Proximal trichorrhexis nodosa has been linked to the use of certain drugs such as trametinib and tumor necrosis factor inhibitors.44,45 Congenital forms of trichorrhexis nodosa also exist and can occur in association with other disorders, such as Menkes syndrome, trichothiodystrophy, monilethrix, congenital arginosuccinic deficiency, citrullinemia, trichohepatoenteric syndrome, biotin deficiency, and hypothyroidism.3,6,7,16,46–48 Congenital trichorrhexis is inherited in an autosomal dominant fashion. Patients typically have normal hair for the first few months of life, but this is then replaced by sparse, fragile hair.17
Bubble HairBubble hair is an acquired hair shaft disorder that is more common in women. Patients usually present with a patch of short, fragile hair.3 Examination by trichoscopy or optical or electron microscopy shows air-filled cavities (vacuoles) in the cortex of the hair shaft that correspond to keratin hydrolysis and local air expansion induced by the passage of hot water through the shaft (Fig. 1H). Bubble hair is associated with the use of hair dryers, curling tongs, and hair straighteners at temperatures above 125 °C.43 It can also be associated with trichorrhexis nodosa and trichoptilosis. The condition can be improved by avoidance of excessive heat and chemicals.43,49
Loose Anagen Hair SyndromeLoose anagen hair syndrome is a hair shaft disorder caused by defective anchoring between the hair shaft and scalp.2 It is more common in girls with blonde hair aged between 3 and 6 years (Fig. 6A), although it has been described in patients of all skin phototypes and in adults. It is associated with mutations in SHOC2 (10q.25) and KRT75.77,78 (12q.13).6
Hair typically comes away easily and painlessly if tugged gently and there may be diffuse hair loss. Parents typically mention that they do not have to cut their children’s hair because of sparse growth.50
LAS has been classified into 3 phenotypes51:
Type A: decreased hair density.
Type B: difficult-to-style hair.
Type C: normal-appearing hair with increased hair loss.
Optical microscopy shows dystrophic anagen bulbs without a sheath (conferring a golf club–like appearance) and proximal ruffling of the cuticle (Fig. 1I) (Fig. 6B).52
Loose anagen hair syndrome resolves spontaneously with time and does not require treatment. Good response has been reported for topical minoxidil 2% and 5%.2,53
FundingThe authors declare that they did not receive any funding for this article.
We thank Dr. Martín-Santiago for her comments during the preparation of this manuscript and for the clinical images provided, Dr. Saus for his dedication and microscopic images, and Marina Cascales for her kind help preparing the graphic representations.
Please cite this article as: Giacaman A, Ferrando J. Claves diagnósticas en displasias pilosas I. Actas Dermosifiliogr. 2022;113:141–149.