Juvenile dermatomyositis is a rare systemic connective tissue disease with onset during childhood. It presents clinically with proximal muscle weakness and characteristic skin involvement. Diagnosis is based on the Bohan and Peter criteria, though many authors are now substituting biopsy with muscle magnetic resonance imaging (MRI) for both diagnosis and follow-up. Without intensive early treatment, complications such as calcinosis cutis and lipodystrophy can develop in the chronic phases of the disease. Early recognition is therefore key to management. We present a series of 5 patients who were diagnosed with Juvenile dermatomyositis on muscle MRI without undergoing muscle biopsy and who received early treatment. We draw attention to the usefulness of muscle MRI for the diagnosis of muscle involvement and to the importance of early initiation of intensive treatment to prevent complications.
La dermatomiositis juvenil es una conectivopatía sistémica infrecuente de aparición en la edad pediátrica. Clínicamente se caracteriza por la presencia de debilidad muscular proximal con afectación cutánea característica. El diagnóstico de la enfermedad se realiza mediante los criterios de Bohan y Peter, si bien recientemente numerosos autores están sustituyendo la realización de biopsia muscular por la resonancia magnética muscular (RMM) para el diagnóstico y seguimiento de la enfermedad.
En fases crónicas de la enfermedad, y sin un tratamiento precoz intensivo, pueden aparecer complicaciones como la calcinosis cutánea o la lipoatrofia, por lo que el reconocimiento temprano de la enfermedad es clave en el manejo.
Presentamos una serie de 5 pacientes diagnosticados de dermatomiositis juvenil mediante RMM sin realizarse biopsia muscular y tratados de forma temprana. Resaltamos la utilidad de la RMM en el diagnóstico de la enfermedad muscular y la importancia de instaurar el tratamiento de forma precoz e intensiva para prevenir las complicaciones.
Juvenile dermatomyositis is an uncommon autoimmune systemic connective tissue disease. Despite its low prevalence, it accounts for 85% of all idiopathic inflammatory myopathies in children, and incidence rates vary between 2 and 4 cases per 1 million children depending on the series.1 Clinically, juvenile dermatomyositis is characterized by muscle weakness and distinctive cutaneous manifestations, which tend to occur as the presenting symptom. On occasions, the disease may affect other organs, such as the gastrointestinal tract, the heart, the lungs, the kidneys, and the eyes.2 Other complications, such as lipotrophy and calcinosis, may appear in late stages of disease, but they have become less common since the introduction of early intensive treatment. Diagnosis of juvenile dermatomyositis has traditionally been based on the criteria published by Bohan and Peter in 1975, but muscle biopsy is gradually being replaced by muscle magnetic resonance imaging (MRI), which is a noninvasive procedure that offers high sensitivity and performs better than muscle biopsy in terms of false negatives as it is not affected by the patchy distribution of disease.3–5 In view of these advantages, several groups have proposed incorporating muscle MRI findings into diagnostic and follow-up criteria.1,5,6
ObjectivesWe performed a retrospective chart review of patients diagnosed with juvenile dermatomyositis at our hospital between January 1999 and December 2015. Our aims were to assess disease course and complications in patients treated with early intensive therapy and to evaluate the use of MRI rather than biopsy for detecting muscle involvement.
ResultsWe studied 5 patients (4 boys and 1 girl) with a mean age at diagnosis of 10.4 years (range, 8-12 years). Time to diagnosis ranged from 2 months to 3 years. All the patients developed the characteristic cutaneous manifestations first. These preceded the onset of muscle involvement by periods ranging from 2 weeks to 3 years. The shortest time to diagnosis was observed in the 2 patients who presented with skin complaints (patients #2 and #4). The physical examination revealed Gottron papules and malar rash in patient #2 and Gottron papules, heliotrope erythema, and psoriasiform plaques on the scalp in #patient 4 (Table 1). The presenting manifestation in the other 3 patients was muscle weakness. All the patients had cutaneous manifestations, the most common of which were Gottron papules/the Gottron sign (present in 5/5 patients), heliotrope erythema (4/5), malar rash (4/5) and periungual telangiectasias (3/5) (Table 1).
Clinical Characteristics of Patients.
| Patient | Sex | Age at Diagnosis, y | Time to Diagnosis | First Symptom | Presenting Complaint | Cutaneous Manifestations | Muscle Weakness | Absa | Biopsy | MRI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 | 24mo | Cutaneous | Weakness | HE, GP, PT, MR, | Scapula and pelvis | Neg | Hyperkeratosis, parakeratosis, perivascular lymphocytic infiltrate, mucin, negative DIF | Edema in paraspinal and upper and lower limb muscles |
| 2 | M | 12 | 3wk | Cutaneous | Skin lesion | GP, MR | Scapula | ANAs | Hyperkeratosis, perivascular infiltrate, negative keratinocytes, mucin, fibrinogen with DIF | Scapular muscle edema |
| 3 | M | 12 | – | Cutaneous | Weakness | HE, GP, PT, MR, PHS | Pelvis | ANAs | Ulceration, parakeratosis, perivascular lymphocytic infiltrate, vascular dilation | Pelvic girdle muscle edema |
| 4 | F | 8 | 1.5mo | Cutaneous | Skin lesion | HE, GP, PT, PP | Scapula | Neg | Hyperkeratosis, papillary dermis edema, perivascular lymphocytic infiltrate, vascular dilation | Right deltoid edema |
| 5 | M | 12 | 24mo | Cutaneous | Weakness | HE, GP, MR, CC | Scapula and pelvis | – | Not available (performed at other hospital) | Proximal muscle atrophy in upper and lower limbs |
Abbreviations: Abs, antibodies; ANA, antinuclear antibodies; CC, calcinosis cutis; DIF, direct immunofluorescence; F, female; GP, Gottron papules; HE, heliotrope erythema; LL, lower limbs; M, male; MR, malar rash; MRI, muscle magnetic resonance imaging; Neg, negative; PHS, photosensitivity; PP, psoriasiform plaques; PT, periungual telangiectasias; UL, upper limbs.
The 5 patients had proximal muscle weakness, elevated levels of 1 or more muscle enzymes (alanine aminotransferase, aspartate aminotransferase, creatine kinase, aldolase), and muscle MRI findings consistent with myositis in the upper and lower limbs (Fig. 1). The electromyogram, performed in 4 patients, showed a myogenic pattern in the biceps and/or quadriceps. Two patients had positive antinuclear antibodies with low titers (1/160). None of the patients underwent muscle biopsy and a skin biopsy was performed in all cases (Table 1).
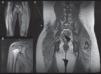
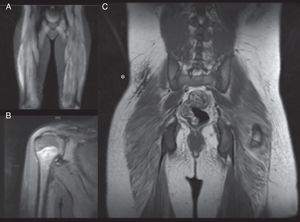
Magnetic resonance imaging (MRI) findings. A, Patient #1. Whole-body muscle MRI. Increased intensity in STIR (short tau inversion recovery) sequence of buttock region and vastus lateralis, consistent with muscle edema, a sign of muscle inflammation. Integration of this finding confirmed the diagnosis of juvenile dermatomyositis. B, Patient #4. MRI of shoulder muscle. Increased signal from right deltoids and fascia in association with a small quantity of liquid in the subacromial bursa. C, patient #5. Whole-body muscle MRI after initiation of treatment. Note the hypointense calcinosis cutis plaque in the right low lumbar region (*).
The starting treatment in 3 patients consisted of oral systemic corticosteroids 1mg/kg/d or intravenous corticosteroids at two 500-mg pulses combined with oral methotrexate 15mg/wk and hydroxychloroquine 200mg/d. Another patient was treated with oral prednisone 1mg/kg/d combined with ciclosporin and 6 boluses of intravenous immunoglobulin (IVIG). The last patient, who had mild muscle involvement, was treated with oral methotrexate 15mg and hydroxychloroquine 200mg combined with topical corticosteroids and tacrolimus. No systemic corticosteroids were administered. Four patients relapsed during follow-up and received different treatments (Table 2). Four patients are currently asymptomatic. Two are not receiving any treatment, 1 is receiving subcutaneous methotrexate 15mg/wk and hydroxychloroquine 200mg/d, and the other is receiving once-daily hydroxychloroquine 200mg as monotherapy (Table 3). None of the patients developed serious complications or organ involvement and just 1 developed calcinosis cutis in the form of a single plaque in the lumbar region 3 years after diagnosis.
Initial Treatment.
| Patient | CORT | MTX | HCQ | TAC | IVIG | CICLO | No. of Relapses/Treatment | Follow-up Duration, y |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 pulses mPDN | 15mg | 200mg | – | – | – | 1/PDN, IVIG, MTX | 3 |
| 2 | Oral PDN | − | − | – | 6 boluses | Yes | 2/deflazacort | 9 |
| 3 | Oral PDN | 15mg | 200mg | – | – | – | 1/tacrolimus | 11 |
| 4 | Topical | 15mg | 200mg | Topical | – | – | 0 | 5 |
| 5 | Oral PDN | 15mg | 200mg | – | 1 bolus | – | 1/PDN, MTX, HCQ, IVIG | 6 |
Abbreviations: CORT, corticosteroids; CICLO, ciclosporin; MTX, methotrexate; HCQ, hydrochloroquine; IVIG, intravenous immunoglobulin; mPDN, methylprednisone; PDN, prednisone; TAC, tacrolimus.
Maintenance Treatment.
| Patient | CORT | MTX | HCQ | TAC | IVIG | CICLO | Current Situation |
|---|---|---|---|---|---|---|---|
| 1 | PDN 30mg 5 mo | 15mg | 200mg | – | 6 boluses | – | Under treatment |
| 2 | – | − | − | Oral | – | Yes | No treatment |
| 3 | – | Tapering regimen | 200mg | – | – | – | Stable with treatment |
| 4 | – | Tapering regimen | 200mg | Topical | – | – | No treatment |
| 5 | – | 20mg | 200mg | – | 7 boluses | – | Stable with treatment |
Abbreviations: CORT, corticosteroids; CICLO, ciclosporin; MTX, methotrexate; HCQ, hydrochloroquine; IVIG, intravenous immunoglobulin; PDN, prednisone; TAC, tacrolimus.
We have presented 5 cases of juvenile dermatomyositis with cutaneous and muscle manifestations but no involvement of the visceral organs. The ratio of male to female patients was 4:1. This rate contrasts with reports of higher proportions of girls in larger series.1,2,7,8 The mean age at diagnosis was 10.4 years and the mean time to diagnosis was 11.25 months. These figures are similar to those described in the literature2,7,8 and indicate that diagnosis is often delayed in patients with juvenile dermatomyositis.
Diagnosis was established in all 5 patients on the basis of cutaneous and/or muscle involvement, elevated levels of 1 of more muscle enzymes, an electromyogram showing a myogenic pattern, and abnormal muscle MRI findings. A muscle biopsy was not needed in any of the cases. While not pathognomonic, edema in T1- and STIR (short tau inversion recovery) MRI sequences has been described as a specific feature of idiopathic inflammatory myopathies and juvenile dermatomyositis. Barsotti et al.9 reported that muscle edema had a sensitivity of 92.3% and a specificity of 83.3% for the diagnosis of inflammatory myopathies. Degree of edema on the MRI has also been found to correlate with levels of disease activity.3,5,9 The pelvis offers the greatest yield in muscle MRI, followed by the buttocks, the vastus medialis and lateralis, and the shoulders, thorax, and neck.5 Several authors have called for the use of whole-body MRI as the imaging test of choice because early involvement of the muscle in the above locations may not be clinically detectable.3,9 Detection of muscle edema by whole-body MRI in these and other locations would enable the early detection of asymptomatic disease, even in patients without elevated muscle enzymes or with a negative muscle biopsy result.3,5
Muscle biopsy can produce false-negative results in juvenile dermatomyositis due to the patchy distribution of disease. Muscle MRI, by contrast, examines the entire muscle area. It, therefore, offers greater sensitivity and, where necessary, can help in the selection of an appropriate muscle biopsy site.3,9 It also has high sensitivity for the detection of recurrence during follow-up, as it can show signs of disease activity in patients with normal muscle enzyme levels.4
Like other authors,1,3,9–12 we believe that muscle MRI is a valuable diagnostic and follow-up tool for juvenile dermatomyositis. It is safe, sensitive, and noninvasive and can be used instead of muscle biopsy in most patients. Several authors have proposed using muscle MRI as an additional diagnostic tool for juvenile dermatomyositis and the procedure is currently used by different working groups to evaluate and monitor inflammatory myopathies.1,3,4,9,12
Four of the 5 patients in our series showed excellent progress over a follow-up period of 4 to 12 years, and they are all currently asymptomatic and free of sequelae. The fifth patient was diagnosed and treated at a hospital in Ecuador for 3 years before being referred to our hospital with calcinosis cutis presenting as a small plaque. The plaque probably developed because the patient did not receive early aggressive treatment. Calcinosis has been linked to the presence of persistent inflammatory activity, and many authors have shown that early initiation of intensive treatment can prevent later complications.7,8,13
Although the prognosis of juvenile dermatomyositis has improved, early initiation of aggressive treatment and long-term follow up are still necessary as the disease runs an unpredictable course.
ConclusionsWe have presented 5 cases of juvenile dermatomyositis, 4 of which were associated with excellent outcomes. These favorable results are consistent with reports in the literature and are probably thanks to the early initiation of intensive therapy.1,6,8,11,14 Just 1 patient developed calcinosis cutis and this was possibly due to late initiation of nonaggressive therapy.
Dermatologists have an important role in diagnosing juvenile dermatomyositis15 and muscle MRI is a safe, sensitive, noninvasive test for detecting and monitoring muscle involvement.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Corral-Magaña O, Bauzá-Alonso AF, Escudero-Góngora MM, Lacruz L, Martín-Santiago A. La resonancia magnética muscular y el tratamiento agresivo precoz, claves en la dermatomiositis juvenil. Actas Dermosifiliogr. 2018;109:e42–e46.