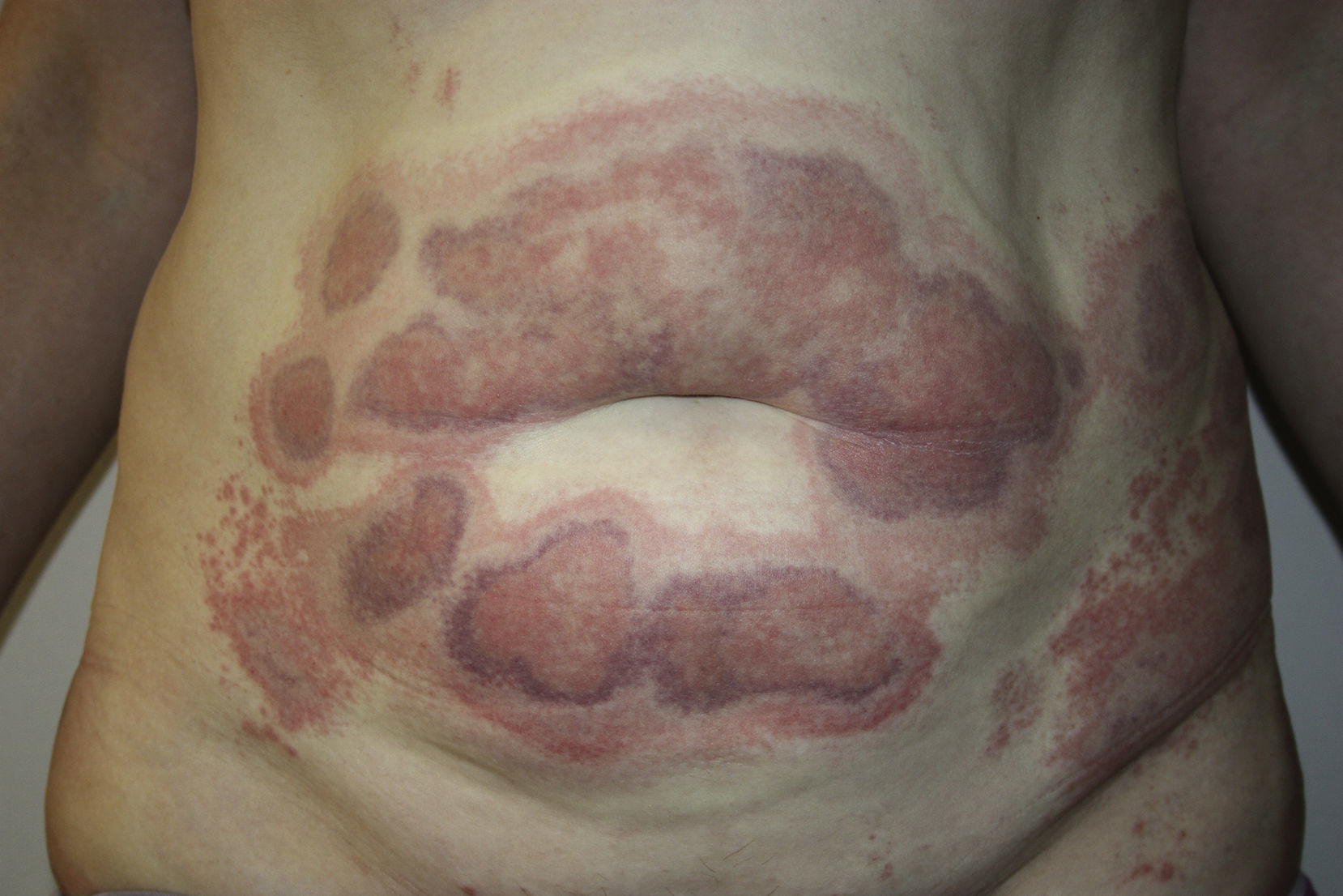
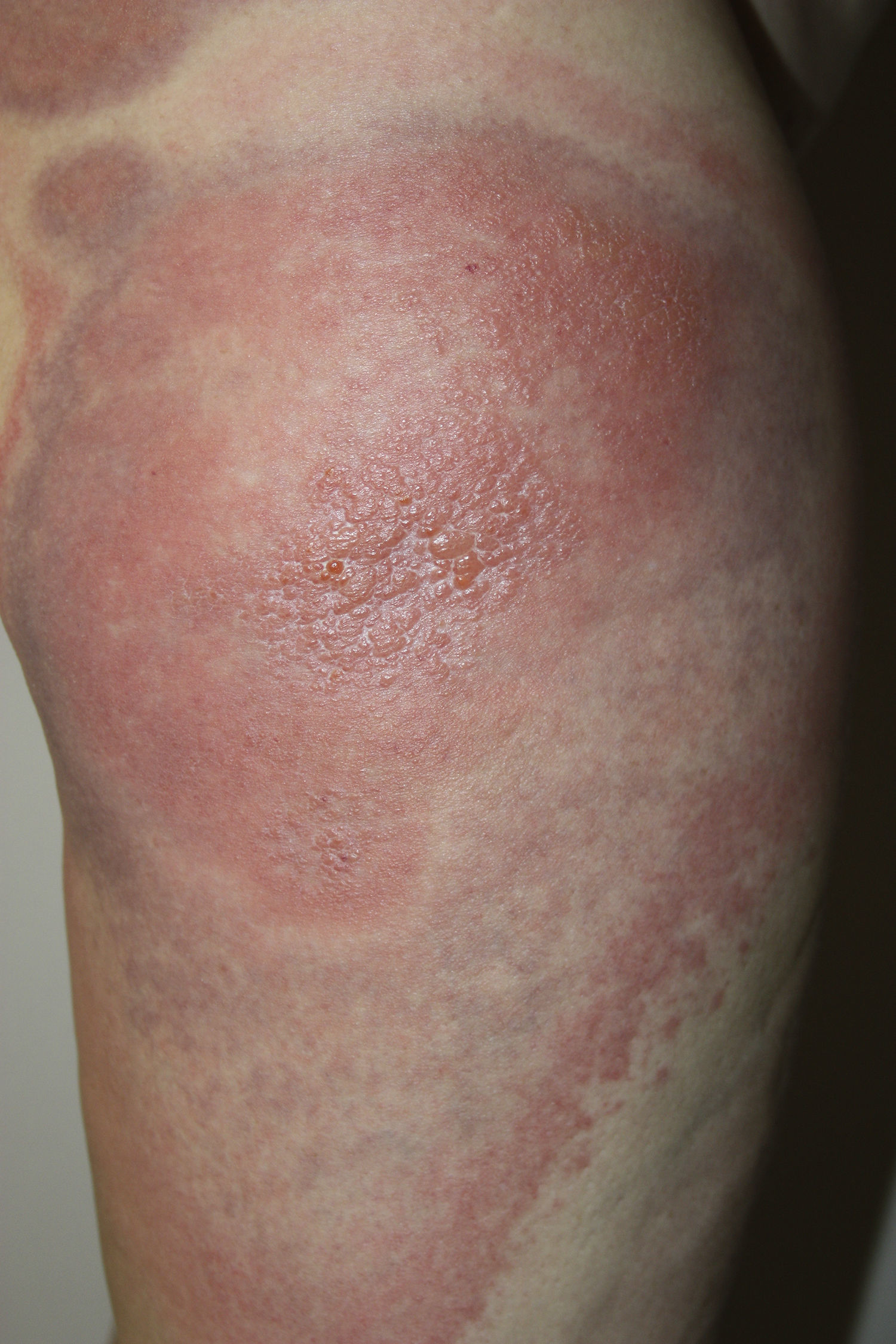
A 45-year-old woman with no past medical history of interest and not on any long-term medication, was seen in the emergency department for the appearance some days earlier of multiple, painful and pruritic, edematous erythematous plaques with purpuric geographic borders. In some areas the lesions were confluent, producing very large plaques (Fig. 1). Some of the lesions had developed superficial vesicles (Fig. 2) despite oral treatment with hydroxyzine, deflazacort, and cefalexin.
Extensive blood tests, including coagulation studies, were performed and were normal. Histology of the lesions revealed intense superficial edema with a moderate perivascular and interstitial inflammatory infiltrate formed of lymphocytes, histiocytes, and numerous eosinophils, occupying the full thickness of the dermis and infiltrating the subcutaneous adipose tissue. Patchy mild spongiosis and hyperkeratosis with foci of parakeratosis were observed in the epidermis. There were no findings suggestive of vasculitis.
After evaluation of the patient in the emergency department, treatment was started with oral prednisone, 1mg/kg/d, plus amoxicillin-clavulanic acid, bilastine, and topical clobetasol. With this treatment, the lesions improved progressively, with desquamation, until their almost complete disappearance within a week, leaving minimal residual pigmentation.
On further questioning, the patient remembered having undergone lipolytic mesotherapy treatment in the affected areas a week before the onset of symptoms. The mesotherapy treatment involved multiple subcutaneous injections of Dermaheal LL (5% phosphatidylcholine, 1% L-carnitine, and biomimetic peptides; Lab Imex, Valencia, Spain), at the sites of appearance of each one of the lesions. The patient denied having used any cosmetic product exclusively in the affected areas.
After resolution of the lesions, patch testing was performed with the standard battery of the Spanish Contact Dermatitis Research Group (GEIDAC), the Marti-Tor cosmetics battery, and the product itself (Dermaheal LL); the results were negative or not relevant in the readings made at 48 and 96hours and at 7 days. Only weak positivity to mercury and thiomersal was observed at 48 and 96hours. The immediate and delayed readings of the prick test and intradermoreaction test with Dermaheal LL were also negative. The patient was not willing to undergo a subcutaneous provocation test.
Mesotherapy or intradermotherapy is a minimally invasive, nonsurgical procedure that consists of multiple intradermal injections of small quantities of various substances in solution, applied directly to the area to be treated.1 The technique was first described in 1958 by Pistor for the treatment of joint and muscle pain. In recent years it has come to be used in cosmetic medicine as a technique for facial rejuvenation and for the treatment of alopecia or areas of hyperpigmentation.2
Lipolytic mesotherapy uses various agents with lipolytic activity (mainly phosphatidylcholine and deoxycholate) for the treatment of localized fat deposits in the neck, eyelids, hips, abdomen, or thighs.3 These substances act as detergents, provoking nonspecific damage to the cell wall, with adipocyte necrosis and subsequent inflammatory infiltrate with a predominance initially of neutrophils and later of T lymphocytes, with granulomas and foam cells.4 The final stage is secondary fibrosis in the adipose tissue.5
Although mesotherapy is considered a safe procedure as it is minimally invasive and is usually well tolerated, numerous local and systemic side effects have been reported. Nausea, vomiting, and dizziness can develop as nonspecific effects of the treatment. Systemic complications include hypersensitivity reactions to the substances administered, hypertransaminasemia, demyelinating neuropathy, and ischemic colitis.2
Mild pain, hematomas, and erythema, and moderate pruritus and edema are often observed locally, at the sites of injection, and tend to resolve untreated within a few days. These manifestations are due to the direct action of the lipolytic substances on the adipocytes. Agents that stimulate the adrenergic receptors, such as isoproterenol or salbutamol, activate cell metabolism, reducing the amount of triglycerides stored in the adipocytes. Mesotherapy with adrenergic stimulants appears to be better tolerated as it does not involve the use of agents with direct toxic effects on the fat cells.6
Granulomas, which may be of infectious7,8 or noninfectious origin,9 scars, atrophy, and lipodystrophy are late complications that can appear after mesotherapy treatment and can often resolve leaving marked and permanent cosmetic alterations.
There is only 1 previous report of the appearance of large erythematous edematous plaques in the areas of injection of lipolytic substances, similar to those observed in our patient.10 The injection of excessive quantities of substances with a lipolytic effect, poor circulation, previous scarring in the area treated, or a poor injection technique that leaves the bolus too superficial, are possible causes of the appearance of this complication.
We have presented a patient with an intense local reaction at the sites of injection of lipolytic mesotherapy, probably due to a direct toxic effect of the substances employed. There are very few reports of this complication in the literature and, considering that mesotherapy is a widely used technique, this may suggest that this type of reaction is underdiagnosed.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Córdobascordoba.hflr@salud.madrid.org S, Rojas E, Garrido-Ríos A, Borbujo J. Reacción local intensa en zonas de inyección de mesoterapia lipolítica. Actas Dermosifiliogr. 2017;108:958–959.