Chronic spontaneous urticaria (CSU) is a disease with a high impact on patients and relatives.1 Studies in France and Germany suggest that the estimated cost generated by pharmacological treatment is around 900–3000dollars/year per person.1 The use of antihistamines in conventional or higher doses allows control in 20–70% of patients and when a therapeutic failure occurs, the use of omalizumab and/or cyclosporine provides an additional 30–40% of control.2 The measures to define control with these treatments use different parameters: quality of life, disease activity, or control perceived by the patient. Scales present a moderate correlation between them (r=0.301–0.501),3,4 but in some cases, discrepancies could lead to different choices at switching antihistamine drug therapy to omalizumab. Recently, the EAACI/GA2LEN/EuroGuiDerm/APAAACI guide5 recommends the Urticaria Control Test (UCT) as the parameter for pharmacological decisions, which is based on the patient perception of control in the last month, replacing the previous recommendation of use UAS7 (Urticaria Activity Score) which considers the activity of the disease in the last week according to the intensity and frequency of the symptoms. Since these two tools have different parameters, “control” definition is also different, and this have implications for the decisions in treatment changes. These choices can have a great impact in health system, especially for economic burden since it may involve the transition from a relatively low-cost and affordable therapy (antihistamines) to a substantially more expensive one (omalizumab), especially in low-middle income countries.
Here, we evaluated the frequency of omalizumab indication according to UAS7, or the UCT. Additionally, we evaluated the frequency of control achieved with omalizumab according to the different scales. These results will explore the impact of these changes from a social cost perspective.
We selected patients with CSU who required the use of top-dose antihistamines (four times the conventional dose), and we evaluated the level of control according to UAS7 and UCT. After at least one month with antihistamines, those patients who according to some of these two scales have not control (UAS7>6 points, UCT<12 points) were treated with omalizumab at dose of 300mg per month and follow-up by 6 months.
Patients were recruited during 2020–2021 for the URTICA cohort from 3 different centers in Colombia. Patients were older than 12 years, with a diagnosis of chronic urticaria according to international guidelines.5 Exclusion criteria were systemic disease presentation that could explain the hives and systemic steroids usage during the last 3 weeks before recruitment or any other therapy that could interfere with the evaluation of symptoms. Patients with only angioedema were excluded. This study was approved by the Ethics Committee of IPS Universitaria Clinics and the University of Antioquia (registry no. BE-IIM 200910 and no. IN13-2013, respectively). All subjects or their legal guardian (younger than 18 years) signed an informed consent approving their voluntary participation in the study.
Statistical analyses were done using SPSS version 26.0 (SPSS Inc, Chicago, Ill) and PRISMA version 9. Total number and proportions were reported for categorical data. Frequency rates and their 95% CIs were obtained. Mann–Whitney U test was used for comparison of continuous variables at baseline and patients with omalizumab after six months. Differences between proportions were analyzed by Pearson chi-square test (or McNemar test when appropriate).
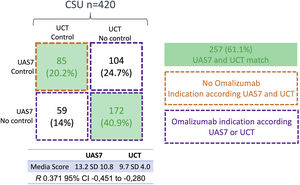
Four-hundred-twenty patients were included (mean age: 47 years, range 16–68; n=300 (71.4%) females). The mean UAS7 was 13.2 points (SD 10.8) and UCT was 9.7 (SD 4). Low and high points in UAS7 and UCT indicates control respectively; the two scales were inversely correlated (r=−0.370; −0.280 to −0.451 95% CI; p<0.001). Both agreed on the decision to administer omalizumab (20.2%) or not (40.9%) in 257 patients (61.1%). With UCT, the need for omalizumab was significantly more frequent than with UAS7 (276 (65.7%) vs 231 (55%) (difference 10.7%, 95% CI 7.6–14.5%; p 0.01). The use of only the UCT to determine the use or not of omalizumab generates an increase in its indication of 10.7%, which could significantly increase spending for the health system. On the contrary, the use of the two scales could reduce this cost and would allow the therapy to be selected in patients who are more inclined to receive it.
A total of 335 (79.7%) patients have indication of omalizumab according UAS7 or UCT (Fig. 1) but 292 patients decided not to receive it: The reasons for not receiving omalizumab were: patient considers not needing omalizumab because considers himself/herself controlled (n=150, 51.3%), logistic barriers (n=72, 24.6%), fears related to the drug or the way of application (n=36, 12.3%), other reasons (n=34, 11.6%). In Colombia, all patients within the general health plan that covers 98% of the population have free access to omalizumab.
Control of patients with CSU according to UAS7 and UCT. “Control” or “No control” was defined according to cut-off in clinimetric scale; UAS7 control<6 points; UCT control>12 points. The two green boxes indicate match between the two scales; the box with an orange line shows the patients who do not have an indication for omalizumab and the three boxes with the purple line shows patients with indication of omalizumab according to one or both scales.
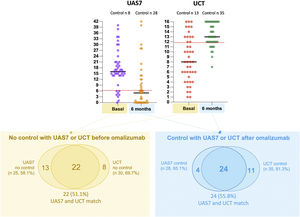
Forty-three patients agreed to receive omalizumab; 35 had indication according UAS7 and 30 according to UCT. After omalizumab, 20 (46.5%) additional patients have control with UAS7 and 22 (51.1%) with UCT. There was not statistically or clinically significant difference in the net benefit of control obtained using either scale for the evaluation of clinical control with omalizumab (20 vs 22: net difference 2 of 43 (4.6%), p 0.3) (Fig. 2).
Clinimetric scales are useful for patient follow-up and decision-making. Other aspects besides disease activity (e.g., patients’ perspective) have become important to define the control of the disease and recently, some guidelines suggest the replacement the UAS7 for UCT to take treatment decisions about pharmacotherapy in urticaria.5 UCT has the advantages over the UAS7 that it considers the patient's perception of the impact of the disease on daily activities. In addition, the UAS7 requires a 7-day evaluation, which often makes it difficult to perform it correctly.
However, according to our results, this change could have an impact on the health system since with the use of UCT there is an increase in the indication of omalizumab, hence a higher cost in health system. The evaluation between the cost of therapies and their benefit is a relevant issue in decision-making especially for health care regulation. In our study a high number of patients preferred not to use omalizumab even though had no control according one of the scales; the majority of those who agreed to use omalizumab presented lack of control according both UAS7 and UCT. Each scale evaluates a specific aspect, and our results indicate the need to evaluate multiple parameters, to better recognize patient's preferences before starting a new therapy. These could result in a reduction of unnecessary treatments with a benefit for the patient and health system.
In conclusion, among CSU patients who use high doses of antihistamines the UCT identifies a greater number of patients requiring omalizumab over UAS7. It is necessary to evaluate the control of the patient from different aspects to define if the benefit obtained justifies the higher cost.
Conflict of interestThe authors declare they have no conflict of interest.