The Spanish guidelines for the treatment of psoriasis recommend that drug choice should be guided by the criteria established in the Summary of Product Characteristics (SPC) for each agent and that decisions should be made on a case-by-case basis and take into account economic considerations.1 Since the introduction of biologic agents, several authors have studied their cost-efficacy.2–4 However, none of these studies present an overall view of the results at clinically significant evaluation time points. The equation used for cost-effectiveness analysis, which can support therapeutic decisions, is the incremental cost effectiveness ratio (ICER): ICER = (C1–C2)/(E1–E2), where C1 and E1 are the cost and effect in the treatment group and C2 and E2 are the cost and effect in the control group.
The present analysis is intended to provide useful information to guide clinical practice based on the results of a recent meta-analysis,5 evaluating the most important variables at each time point. The outcomes and time points used were as follows: a 50% reduction in PASI score (PASI 50) at the time point the SPC recommends assessment of response in order to identify primary treatment failure, and PASI 50 and PASI 75 responses at week 24 (the end of the induction phase). The cost of each treatment was calculated on the basis of the pharmaceutical company's sale price as of January 2014 less the mandatory deduction under Spanish law (Real Decreto 08/2010) plus VAT.6 In the case of infliximab, the cost was calculated for a patient weighing 80 kg and included the additional expense of intravenous infusion at the updated price published by the SOIKOS-OBLIKUE program for 2014 (€254.68).7 No other additional costs were included in the analysis. The ICER was calculated by dividing the cost of treatment up to the time point when response should be assessed by the mean incremental efficacy (drug minus placebo). ICER 95% CI were calculated using the lower and upper 95% CI limits of the incremental efficacy values published in the meta-analysis.5
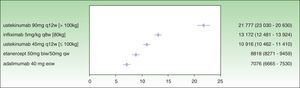
Analysis of incremental cost-effectiveness for PASI 50 at the time point recommended in the SPC for treatment response assessment to identify nonresponders (week 12 for etanercept, week 16 for adalimumab, week 22 for infliximab, and week 28 for ustekinumab) shows that the drug with the lowest ICER is adalimumab (€7076), followed by etanercept (€8818), ustekinumab 45 mg (€10 916), infliximab (€13 172), and ustekinumab 90 mg (€21 777) (Fig. 1.)
While the analysis was carried out at the time point recommended in the SPC for the assessment of primary response, the treatment had not, in some cases, reached maximum efficacy. Owing to this difference in time to maximum response and the large differences in the number of weeks specified before treatment response assessment for each drug (16 weeks between ustekinumab and etanercept, assessed at week 28 and 12, respectively), imputing the cost-efficacy of treatments at these very different time points does not result in an equitable analysis. The same reasoning can be applied to the analysis of the ICER for the time point at which the PASI 75 response is assessed to provide registry data for each drug.
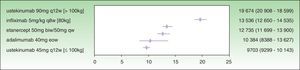
The assessment of PASI response at week 24 is a very useful variable in clinical practice because the decision concerning the success or failure of treatment can be made at that time. By week 24, the induction phase has been completed in all the biologic agents and efficacy tends to have reached a plateau.7 Moreover, analysis at this time implies calculating the cost of treatment at the same point for all the drugs. The ICER results for a PASI 50 response at week 24 are as follows: ustekinumab 45 mg (€9703), followed by adalimumab (€10 384€), etanercept (€12 735€), infliximab (€13 536), and ustekinumab 90 mg (€19 674 ) (Fig. 2). As shown in Fig. 2, the 95% CIs of the ICERs for etanercept and infliximab overlap considerably, both one with another and with adalimumab. It is therefore not possible to establish any significant differences.
The results for a PASI 75 response at week 24 show that the treatment with the lowest ICER is ustekinumab 45 mg (€10 371), followed by adalimumab (€10 549 ), infliximab (€14 514 €), etanercept (€16 080 ), and ustekinumab 90 mg (€20 880 ) (Fig. 3). In this case, the 95% CIs for ustekinumab 45 mg and adalimumab almost overlap, and both are significantly favorable compared to infliximab, etanercept, and ustekinumab 90 mg.
To stratify the data on treatment with ustekinumab, we used the weight distribution of patients with psoriasis in the Spanish BIOBADADERM registry.8According to this data, 9.25% of these patients weigh over 100 kg. If we estimate that half of these patients are treated with ustekinumab 45 mg and half with ustekinumab 90 mg (in some regions this regimen is not reimbursed), the ICER values at week 24 for ustekinumab adjusted for weight distribution would be as follows: PASI 50, €10 189 and PASI 75 €10 994. When the ICER for PASI 50 at week 24 is evaluated, ustekinumab adjusted for weight distribution is still the most cost-effective treatment, while in the case of PASI 75 it is the second most cost-effective treatment, coming after adalimumab. In both cases, the 95% CI for ustekinumab adjusted for weight distribution overlaps that of adalimumab, meaning that the numerical differences would not be significant. In all cases, the calculations performed represent an extrapolation which, despite its limitations, approximates routine clinical practice.
As psoriasis is a chronic disease, long-term results should be included in the analysis, including the additional cost of combining, escalating, or switching treatments in patients who do not achieve an optimal response or experience a loss of initial response over time, as well as the potential savings in patients in whom treatment intensity can be reduced or an intermittent regimen can be used.
However, the cost of the induction phase before a population-based plateau of treatment response is achieved represents an important component of the total cost of treatment in the first year. The present analysis may be useful as regards treatment decisions, which must always be made on a case-by-case basis.
Please cite this article as: Puig L, López-Ferrer A, Vilarrasa E. Análisis de coste-eficacia incremental de los tratamientos biológicos para la psoriasis en los momentos de valoración significativos para la práctica clínica. Actas Dermosifiliogr. 2014;105:951–953.