Reflectance confocal microscopy (RCM) is a novel tool for noninvasive in vivo diagnosis of nonmelanoma skin cancer at high resolution. Given the excellent correlation that has been demonstrated between RCM findings and routine histology, clinicians using this imaging technique can establish an immediate diagnosis. Multiple studies have evaluated the use of RCM in melanoma and nonmelanoma skin cancer and have demonstrated high sensitivity and specificity rates. We discuss the applicability of RCM for the early diagnosis of NMSC and discuss other clinical applications of this emerging technique.
La microscopía confocal de reflectancia (MCR) es un herramienta novedosa para el diagnóstico precoz in vivo del cáncer de piel no melanoma (CPNM) con alta resolución. Dada la excelente correlación demostrada entre los hallazgos de la MCR y la histología de rutina, los clínicos que utilizan esta técnica de imágenes pueden establecer un diagnóstico inmediato. Múltiples estudios han evaluado el uso de la MCR en cáncer de piel melanoma y no melanoma, demostrando altas tasas de sensibilidad y especificad. Hablamos de la aplicabilidad de la MCR para el diagnóstico temprano de CPNM, comentando otros aplicaciones clínicas de esta técnica emergente.
Nonmelanoma skin cancers (NMSC) are the most common tumors in the white population, with rising incidence rates reported in recent decades.1–4 While the term NMSC encompasses a wide variety of cutaneous malignancies, the most frequent subtypes are basal cell carcinoma (BCC), actinic keratosis (AK), Bowen disease, and invasive squamous cell carcinoma. Overall, most NMSC have an excellent prognosis when diagnosed and treated at an early stage. In routine practice, diagnosis is based on clinical evaluation plus histologic examination of a biopsy specimen. Whereas surgical excision represents the treatment of choice for invasive NMSC, novel noninvasive treatments may be applied in the case of AK and superficial variants of BCC.
Clinical diagnosis of a fully developed BCC or squamous cell carcinoma usually poses no major problem for an experienced dermatologist. However, diagnosis can be complicated in patients with multiple or very small lesions and in the presence of extensive sun-damage. Furthermore, cases in immunosuppressed patients, particularly those receiving systemic immunosuppression therapy after solid organ transplantation, may pose a significant challenge for the clinician as such patients often develop multiple NMSC in addition to wart-like lesions and sebaceous hyperplasia.5 Wart-like lesions in organ transplant recipients can correspond histologically to flat viral warts, seborrheic keratosis, or AK, but are often clinically indistinguishable.
In the past decade, several noninvasive diagnostic tools for the diagnosis of malignant melanoma and NMSC have been developed and studied.6–9 Among these, reflectance confocal microscopy (RCM) offers the possibility of obtaining real-time images of the skin at high resolution, thereby facilitating in vivo assessment of cellular morphology. Consequently, it has been proposed that RCM represents an excellent tool for the early diagnosis and subsequent monitoring of NMSC.
Materials and methodsPatients and clinical evaluationPatients were recruited from an outpatient clinic for NMSC at the Skin Cancer Center Charité (Charité – Universitätsmedizin Berlin). Informed consent was obtained from the patients and all research was conducted according to the principles of the Declaration of Helsinki.
In vivo reflectance confocal microscopyIn vivo RCM was performed with a commercially available device (Vivascope 1500, Lucid Inc, Rochester NY, USA; Mavig GmbH, München, Germany). A detailed description of this device and the technique used has previously been published.9–11 Briefly, the device is equipped with an 830nm diode laser operating at low power (<20mW) for tissue illumination. The optical principle of this device is based on the detection of reflected and backscattered light from the skin site under investigation. Images are presented in grayscale and image brightness corresponds to the variable degree of reflection (different refraction indices) of each skin component. Reflected light is collected via a small pinhole enabling high-resolution images with a lateral resolution of approximately 1μm and an axial resolution of approximately 3–5μm. Vertical displacement of the lens changes the depth of the image within the tissue, and the system software facilitates the collection of sequential XY planes of the sample. In this way, thin sections of horizontal tissue can be assessed in vivo and the results can then be used to create a three-dimensional reconstruction of the entire area under study.
RCM evaluation protocolFor the purpose of this report, RCM was used to systematically evaluate lesions found to be suspicious for NMSC on clinical examination. The Vivablock horizontal mapping function was used at the level of the stratum spinosum and the superficial dermis (4mm×4mm to 6mm×6mm). In addition, vertical mapping using the Vivastack function was performed in 5μm steps to a maximum depth of 200μm, beginning at the stratum corneum and continuing throughout the entire epidermis and into the papillary dermis. At areas of special interest individual images were captured and stored during the imaging process.
RCM features of NMSCBasal cell carcinomaVarious studies have described the main features of BCC on RCM evaluation, and a large multicenter study has shown high sensitivity and specificity rates for selected RCM parameters12. Although different subtypes of BCC can show varying morphology on RCM, a phenomenon also observed in histology, they share certain common findings that easily allow for the diagnosis of BCC. These include the presence of a uniform population of tumor cells with elongated nuclei oriented along a common axis, a phenomenon that has also been described as nuclear polarization or streaming.13,14 Tumor islands in the dermis visualized as areas appearing less bright than the surrounding stroma have been referred to as hyporefractile tumor islands. Cells on the periphery of the tumor islands show elongated nuclei with peripheral palisading. The overlying epidermis of the BCC often displays a disarranged keratinocyte pattern, probably the result of chronic actinic damage.15 Abnormal blood flow characterized by an increase in the number and diameter of blood vessels may also be observed in BCC; the blood vessels are frequently observed to branch and intertwine between the tumor aggregates and are probably the result of neoplastic angiogenesis. On RCM, blood vessels can easily be identified as parallel arrays and blood flow can be observed during in vivo examination. Another feature seen is the rolling of small round cells along the vessel walls, a phenomenon sometimes referred to as leukocyte rolling. The area surrounding the neoplastic aggregates consists of collagen and inflammatory cells and appears bright on RCM evaluation. The characteristics of pigmented BCC are similar to those of the nonpigmented form. However, in the case of the pigmented subtype, hyperrefractile dendritic cells may be observed in the tumor islands (corresponding to melanocytes) and plump bright cells may be seen in the tumoral stroma (corresponding to melanophages) (Figs. 1 and 2) (Table 1).16
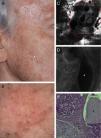
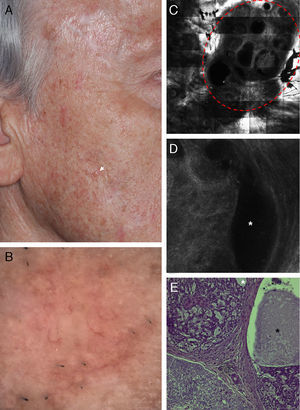
Correlation of clinical, dermoscopic, and reflectance confocal microscopy (RCM) images of a cystic basal cell carcinoma (BCC). (A) Clinical image of the lesion showing severe actinic damage with multiple actinic keratoses. On the right cheek a small skin-colored papule is observed (white arrow). (B) Dermatoscopic image of the lesion showing some telangiectasias around the papule. (C) RCM horizontal mosaic (4mm×4mm) obtained at the level of the superficial dermis showing multiple, round hyporefractile and non-refractile areas in a lobular arrangement (red circle). (D) Single RCM image (0.5mm×0.5mm) showing tumor islands with bright homogeneous centers surrounded by large dark clefts (white asterisk). (E) Histopathologic correlate showing a cystic variant of a BCC with basaloid tumor cells arranged in nests. Areas of mucin deposition (white asterisk) can be observed surrounding the tumor islands and forming cystic spaces (black asterisk).
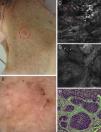
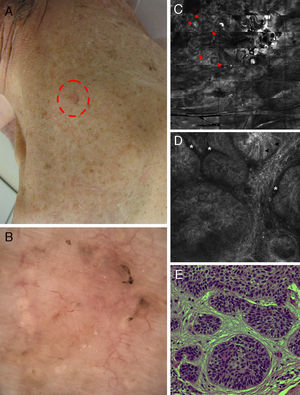
Correlation of clinical, dermoscopic, and reflectance confocal microscopy (RCM) images of a pigmented basal cell carcinoma (BCC) in an immunosuppressed patient after kidney transplantation. (A) Clinical image showing a flat erythematous plaque on the neck with brown pigmentation around the periphery of the lesion. (B) Dermoscopy reveals fine telangiectasias in the center and brown pigmentation at the border of the lesion. (C) RCM mosaic obtained at the level of the superficial dermis (4mm×4mm) shows multiple hyporefractile tumors islands in the upper dermis (red arrowheads). (D) On a single RCM image (0.5mm×0.5mm) these tumor islands display elongated nuclei with peripheral palisading (black arrowheads) and are separated from the surrounding stroma by dark, cleft-like spaces (white asterisk). (E) Hematoxylin–eosin histology shows tumor nodules comprising basaloid tumor cells with peripheral palisading and surrounding fibrous stroma.
Key features of basal cell carcinoma (BCC) on reflectance confocal microscopy (RCM).
| RCM features of BCC |
| Stratum corneum |
| • Superficial disruption |
| • Amorphous grey material (crusting/bleeding) |
| • Neutrophils and/or impetiginization |
| Stratum granulosum/spinosum |
| • Mild keratinocyte atypia (atypical honeycomb pattern) |
| • Presence of dendritic cells |
| Dermoepidermal junction |
| • Elongated keratinocytes and nuclei |
| • Orientation of nuclei along the same axis (polarization) |
| Dermis |
| • Homogeneous tumor islands of lower reflectance than the surrounding stroma |
| • Palisading of elongated cells at the periphery of the tumor nodules |
| • Dark clefts between tumor islands and surrounding fibrous stroma (corresponding to mucin deposition) |
| • Small bright cells and dendritic cells/inflammatory infiltrate |
| • Increased vascularity: |
| - increase in the number and tortuosity of blood vessels |
| - large caliber blood vessels |
| - leukocyte trafficking |
Actinic keratoses (AK) represent a proliferation of a typical keratinocytes limited to the epidermis. Depending on the extent of the atypia present, 3 different grades of AK have been described.17 On RCM evaluation, AK displays several features that facilitate a noninvasive diagnosis. Superficial disruption of the stratum corneum with detached keratinocytes is observed; these cells, unlike those found in normal skin, often contain nuclei that correspond histologically to parakeratosis. At the level of the stratum granulosum and spinosum, keratinocyte atypia appears as pleomorphism of single cells with variation in their size and shape. On RCM images this results in a characteristic appearance often called an atypical honeycomb pattern. At the level of the superficial dermis, small round-to-slightly elongated blood vessels may be observed. RCM also visualizes changes in the dermal stroma in the form of thickened fibers, which on histologic examination correspond to solar elastosis (Fig. 3).
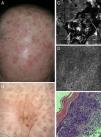
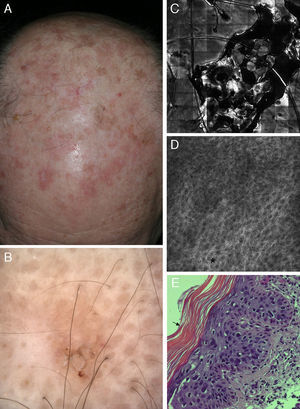
Correlation of clinical, dermoscopic, and reflectance confocal microscopy (RCM) images of an actinic keratosis (AK). (A) Clinical image reveals multiple erythematous to brown hyperkeratotic plaques on the scalp. (B) Dermoscopy reveals a brownish erythematous plaque with central scale lacking the classical strawberry pattern typical of AK. (C) RCM mosaic of the lesions obtained at the granular and spinous layer shows a central area of hyperkeratosis with amorphous material of medium to high reflectance. (D) RCM single image obtained at the periphery of the lesion demonstrates a broadened (black asterisk) and generally atypical honeycomb pattern with variations in cell and nuclei sizes and shapes. (E) Corresponding hematoxylin–eosin histology illustrates overlying hyperparakeratosis (black arrow) and epidermal proliferation of atypical keratinocytes.
Actinic cheilitis, which is the equivalent of AK on the lip, shows similar findings on RCM evaluation. A recent study showed the usefulness of RCM for distinguishing actinic cheilitis from inflammatory forms of cheilitis and also for in vivo monitoring of response to treatment in these patients18 (Table 2).
Key features of actinic keratosis on reflectance confocal microscopy (RCM).
| RCM features of actinic keratosis |
| Stratum corneum: |
| • Superficial disruption with large single keratinocytes |
| • Nucleated cells (parakeratosis) |
| • Small bright cells with dark centers (neutrophils/impetiginization) |
| Stratum granulosum/spinosum: |
| • Atypical honeycomb pattern: variations in cell size and morphology |
| • Broadened honeycomb pattern: areas with broadened and blurred intercellular connections |
| • Loss of regular epidermal architecture |
| Dermis |
| • Slightly dilated blood vessels |
| • Solar elastosis: moderately refractive lace-like material adjacent to collagen bundles |
RCM findings in Bowen disease are similar to those observed in AK, including superficial disruption, an atypical honeycomb pattern, and solar elastosis. However, in Bowen disease atypia and pleomorphism is usually more severe, involving the entire epidermis. Furthermore, large cells with bright centers, which correspond to histologically dyskeratotic cells, are commonly seen in Bowen disease. Multinucleated cells may also be observed. The vasculature in Bowen disease shows characteristic round to S-shaped vessels in the papillary dermis (Fig. 4).19
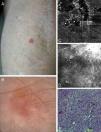
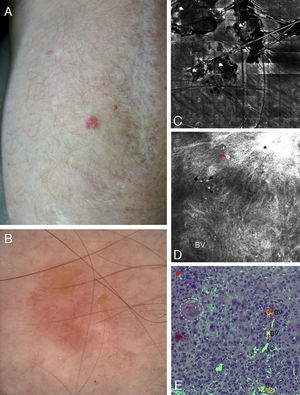
Correlation of clinical, dermoscopic, and reflectance confocal microscopy (RCM) images of Bowen's disease. (A) Clinical image showing a single small erythematous plaque on the forearm in a kidney transplant patient. (B) Dermoscopic examination reveals the presence of glomerular vessels and areas of erosion. (C) RCM mosaic illustrates correlation between erosion and superficial disruption and crusting seen as deposits of low refractile amorphous material (white arrowheads). (D) RCM single images obtained at the level of the stratum spinosum showing complete architectural disruption with atypical honeycomb pattern and broadened honeycomb pattern (black asterisk). Single large cells with bright centers can be observed (red arrowhead), probably corresponding to dyskeratotic cells. Also visualized are small round bright structures that correspond to inflammatory cells. The dilated round-to-oval blood vessels are the RCM equivalent of the glomerular vessels seen on dermoscopy. (E) Corresponding hematoxylin–eosin histology showing marked keratinocyte atypia with large atypical cells, multinucleated cells (white arrowhead) and dyskeratotic cells (red arrowhead). Keratin pearls (white dashed circle) and dilated blood vessels are also visualized.
The RCM evaluation of invasive squamous cell carcinoma may be challenging even for an experienced operator as the hyperkeratosis in these tumors limits the visualization of the deeper layers. In lesions with severe hyperkeratosis, a significant part of the light is backscattered due to the high refractive index of keratin. This results in fuzzy images and impaired visualization of deeper structures. In contrast to AK, in which the superficial hyperkeratosis can easily be removed, the hyperkeratosis in squamous cell carcinomas is usually adherent and attempts to remove the keratotic scale may result in bleeding or pain. However, particularly small evolving squamous cell carcinoma lesions may be evaluated and distinguished from AK by RCM. At the level of the stratum corneum, hyperkeratosis and impetiginization associated with the presence of neutrophils are commonly present. Possible findings in the granular and spinous layers include a variable degree of atypical keratinocytes seen as an atypical honeycomb pattern and, in some cases, the presence of dyskeratotic cells with targetoid appearance. At the level of the superficial dermis, RCM images may visualize tumor islands lacking the features typical of BCC, in particular palisading with elongated cells. Large bright structures corresponding to keratin pearls may be observed within tumor islands and elongated blood vessels are present in the surrounding areas.
Monitoring treatment of NMSCRCM offers the possibility of in vivo monitoring of a skin lesion over time because no tissue is removed or altered during the process. This makes the technique useful for assessing treatment efficacy and for evaluating treatment effects such as necrosis, apoptosis, and the induction of inflammatory infiltrate. In a previous study by our group, RCM was used to monitor the treatment of AK with the topical immune modulator imiquimod. In that study we detected the presence of dendritic-shaped Langerhans cells and other inflammatory cells within AK lesions 2 weeks after start of treatment.20 Re-arrangement of the epidermal structure was observed 4 weeks after cessation of treatment. Similar findings have also been reported for imiquimod treatment in BCC.21,22 The technique may also help to identify nonresponders at an early stage and guide treatment selection because RCM can detect the presence of an inflammatory infiltrate in vivo.
Clinical applications of RCM include the determination of surgical margins in clinically ill-defined lesions, such as amelanotic lentigo maligna of the face.23,24 A recent study has also demonstrated the feasibility of using RCM imaging to identify residual tumor in shave biopsy wounds25 and Ahlgrimm-Siess et al. studied the usefulness of this novel technique for monitoring treatment efficacy after cryotherapy of BCC.26
DiscussionIn vivo RCM is an emerging technology in dermatology that allows rapid in vivo diagnosis of skin cancer and differentiation between benign and malignant lesions. RCM enables high-resolution skin analysis with visualization of morphological details on a cellular level. In this regard, RCM is particularly useful for evaluating suspicious lesions that do not clearly fulfil clinical criteria for nonmelanoma skin cancer on physical examination or have the characteristic dermoscopic features. As a completely noninvasive device, RCM is an excellent tool for the detection of skin cancer in cosmetically sensitive areas of the face and it can also facilitate differentiation between malignant tumors and benign lesions such as sebaceous hyperplasia and seborrheic keratosis.27 However, RCM evaluation of hyperkeratotic lesions, such as warts and squamous cell carcinoma, is limited because of the high refractive index of keratin and the consequent backscattering of light and reduced resolution. Moreover, RCM is more useful in the evaluation of selected lesions which are suspicious on clinical examination because the assessment of multiple lesions would be quite time consuming.
The most obvious advantage of the technique is that diagnoses can be obtained in vivo during the course of the examination. Based on RCM findings, a decision for further management and therapy can be made without delay while at the same time avoiding unnecessary biopsies.
In the context of novel therapeutic modalities for the treatment of NMSC, RCM allows noninvasive monitoring of photodynamic therapy and of topical therapies, such as imiquimod and diclofenac plus hyaluronic acid. RCM can be used to obtain a diagnosis prior to the start of therapy and to verify treatment efficacy during treatment and follow-up. This approach may ultimately decrease the number of procedural complications and increase complete clearance rates compared to clinical evaluation alone.
On the basis of the available evidence, in vivo RCM is a promising and innovative technology for the early diagnosis of skin cancer, which may ultimately play an important role in skin cancer screening and prevention as well as in the early detection of progression or recurrence after therapy.
Conflicts of interestThe authors declare that they have no conflicts of interest.