Antimicrobial photodynamic therapy combines the use of a photosensitizing drug with light and oxygen to eradicate pathogens. Trichophyton mentagrophytes is a dermatophytic fungus able to invade the skin and keratinized tissues. We have investigated the use of new methylene blue as the photosensitizing agent for antimicrobial photodynamic therapy to produce the in vitro inactivation of T mentagrophytes.
Material and methodsA full factorial design was employed to optimize the parameters for photoinactivation of the dermatophyte. The parameters studied were new methylene blue concentration, contact time between the photosensitizing agent and the fungus prior to light treatment, and the fluence of red light (wavelength, 620–645nm) applied.
ResultsThe minimum concentration of new methylene blue necessary to induce the death of all T. mentagrophytes cells in the initial suspension (approximate concentration, 106 colony forming units per milliliter) was 50μM for a fluence of 81J/cm2 after a contact time of 10minutes with the photosensitizing-agent. Increasing the concentration to 100μM allowed the fluence to be decreased to 9J/cm2.
ConclusionsComparison of our data with other published data shows that the susceptibility of T. mentagrophytes to antimicrobial photodynamic therapy with new methylene blue is strain-dependent. New methylene blue is a photosensitizing agent that should be considered for the treatment of fungal skin infections caused by this dermatophyte.
La terapia fotodinámica antimicrobiana combina el uso de un fármaco fotosensibilizante, la luz y el oxígeno para erradicar microorganismos patógenos. Trichophyton mentagrophytes es un hongo dermatofito capaz de invadir la piel y tejidos queratinizados. El objetivo de este trabajo es aplicar la terapia fotodinámica antimicrobiana para la inactivación in vitro de T. mentagrophytes utilizando el nuevo azul de metileno como agente fotosensibilizador.
Material y métodosSe aplica un diseño factorial completo para optimizar los parámetros que permiten la fotoinactivación del dermatofito. Se tiene en cuenta la concentración del nuevo azul de metileno, el tiempo de contacto entre el fotosensibilizador y el hongo antes del tratamiento con luz y la fluencia de luz roja aplicada entre 620 y 645nm.
ResultadosLa mínima concentración de nuevo azul de metileno que produce una mortalidad de todas las células de T. mentagrophytes de la suspensión inicial (concentración ∼106 ufc/ml) es 50μM para una fluencia de 81J/cm-2 y un tiempo previo de contacto hongo-fotosensibilizador de 10min. Si se aumenta la concentración a 100μM la fluencia que se necesita disminuye a 9J/cm-2.
ConclusionesLa comparación de nuestros datos con otros publicados muestra que la susceptibilidad de T. mentagrophytes a la terapia fotodinámica antimicrobiana con nuevo azul de metileno es cepa-dependiente. El nuevo azul de metileno es un fotosensibilizador a tener en cuenta para el tratamiento de las micosis cutáneas causadas por este dermatofito.
Antimicrobial photodynamic therapy (aPDT) eliminates pathogens using visible light in combination with a photoactivatable drug. The interaction of this photosensitizer (PS) with light and oxygen induces the formation of reactive oxygen species (ROS) that kill the infectious agents targeted for eradication. Because no specific cellular target is used, it is very difficult for cells to develop resistance to the PS used.1aPDT is especially appealing because of its selectivity, which is a consequence of the use of PSs with a preferential affinity for microbial cells over host tissue and the fact that the photodynamic effect is restricted to the light-treated area.2
Trichophyton mentagrophytes is a dermatophytic fungus that invades the stratum corneum of the epidermis as well as keratinized structures such as hair and nails, causing cutaneous mycoses commonly known as tineas.3,4 Most current treatments are based on the use of antifungal agents, which can cause undesirable side effects, especially when they must be taken orally.5
New methylene blue (NMB) is a planar tricyclic phenothiazinium that has phototherapeutic potential due to the fact that its absorption wavelength is in the range of maximum penetration of light in the host tissue (600-850nm).6,7 In aqueous buffer solutions, NMB absorbs light at 630nm and emits fluorescence at 650nm.
The aim of this study was to optimize the use of aPDT with NMB as the PS for the in vitro photoinactivation of T. mentagrophytes.
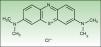
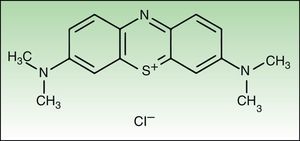
Materials and MethodsChemical ReagentsNMB and sterile Dulbecco's phosphate-buffered saline (PBS) with a pH of 7.4 were acquired from Sigma-Aldrich, Inc. (St. Louis, MO, United States). Fig. 1 shows the structure of NMB.
The fungal culture medium, Sabouraud agar (SA), was prepared from Sabouraud dextrose agar with a pH of 5.6±0.2 (code CM0041, Oxoid Limited, Basingstoke, United Kingdom).
A stock solution of NMB 1mM was prepared in PBS (pH 7.4). For the working solutions, the NMB solution was dissolved in PBS in darkness until the desired concentration was reached and stored at 4°C.
Fungal Strain, Culture Conditions, and Preparation of Cell SuspensionsT. mentagrophytes CECT 2956 were obtained from the Spanish Collection of Type Cultures (Valencia, Spain).
T. mewntagrophytes CETC 2956 cultures were grown on SA and incubated in the dark at 26°C for 14 days. Once the colonies had grown, they were detached from the surface of the solid culture medium by manual agitation in sterile saline solution with 3mm glass balls. The cell suspension was filtered through sterile gauze. The working suspensions were adjusted to a concentration level of approximately 106 colony-forming units (CFU)/mL in sterile saline solution.
Light SourceCell suspensions of T. mentagrophytes were irradiated with a Photocare light-emitting diode (LED) lamp (Sorisa, Sant Quirze del Vallès, Spain). To excite the NMB, the lamp was set to emit red light at between 620 and 645±10nm.
Experimental Design to Optimize the Photoinactivation Protocol for the Trichophyton mentagrophytes DermatophyteThe protocol for photoinactivation of T. mentagrophytes cells by aPDT was optimized using the full experimental design derived from the combination of 3 variables applied at different levels: final NMB concentration (0, 0.1, 1, 10, 25, 50, 75, 100, and 1000μM), incubation time of the PS in contact with the fungus (0, 10, 30, and 90min), and irradiation time (0, 10, 30, and 90min).
Table 1 lists the experiments carried out. In summary, 16 experiments were carried out for each of the 9 NMB concentrations tested, for a total of 144 experiments.
Experimental Design for Each Concentration of New Methylene Blue (0, 0.1, 1, 10, 25, 50, 75, 100, and 1000μM).
| Irradiation Time | ||||
|---|---|---|---|---|
| 0 min | 10 min | 30 min | 90 min | |
| Incubation time | ||||
| 0min | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 |
| 10 min | Experiment 5 | Experiment 6 | Experiment 7 | Experiment 8 |
| 30 min | Experiment 9 | Experiment 10 | Experiment 11 | Experiment 12 |
| 90 min | Experiment 13 | Experiment 14 | Experiment 15 | Experiment 16 |
NMB at one of the concentrations specified above was added to each working suspension (approximate concentration 106 CFU/mL) and incubated for 10, 30, or 90minutes at 30°C and 120rpm. After incubation, without previously washing out the excess PS, the samples were irradiated at 620-645nm using a light source for 10, 30, or 90minutes, with a fluence of 9, 27, and 81J/cm−2, respectively, in order to excite the 630nm band of the NMB.
After being treated with light, 0.1mL of each suspension was seeded in Petri dishes containing SA and incubated in the dark at 26°C for 14 days. The colonies formed after the photodynamic treatment were then counted.
For each test, controls for light toxicity (without PS) and for PS toxicity (without light) were carried out under the same experimental conditions.
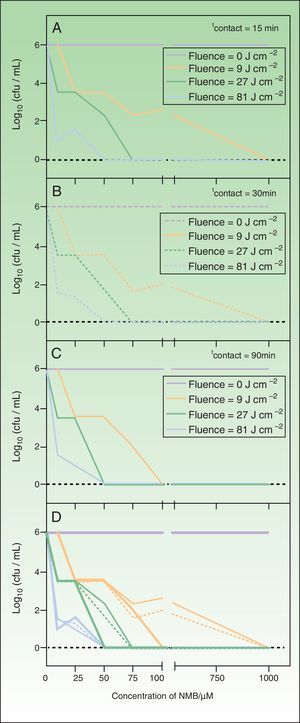
ResultsThe in vitro optimization of the factors used in aPDT with NMB against T. mentagrophytes (PS concentration, contact time, and irradiation time) were assessed according to the decrease in cell viability, expressed as a logarithmic reduction of the number of CFU/mL in relation to the initial cell suspensions of approximately 106CFU/mL. In the absence of PS, exposure of the fungus to light did not inhibit fungal growth at any of the 3 light fluences used (data not shown). The experiment to assess the toxicity of NMB in the dark showed that this PS is only toxic to the dermatophyte when combined with light (red function in Fig. 2).
Logarithmic reduction of the number of colony-forming units per mL of Trichophyton mentagrophytes after photodynamic therapy with new methylene blue, in which the sample was irradiated at 620-645nm.
A, contact time 10minutes. B, contact time 30minutes.C, contact time 90minutes. In all panels, the red function indicates the control light; green function, fluence 9J/cm−2; pink function, fluence 27J/cm−2; blue function, fluence 81J/cm−2. D, summary of results for all contact times tested (thin line, 10minutes; broken line, 30minutes; thick line, 90minutes).
The results shown in Fig. 2 indicate that concentrations of 0, 0.1, and 1μM of NMB did not have a fungicidal effect on T. mentagrophytes; a confluent culture was obtained after irradiation of the sample for all incubation and irradiation times tested. However, a growth-inhibiting effect was detected with NMB concentrations of 10 and 25μM. Under some conditions, the reduction in the number CFU/mL was as large as 5 logarithmic units, but complete inhibition of the fungus was not achieved in any case. Eradication of all colonies was achieved using higher PS concentrations (50, 75, and 100μM) combined with longer incubation and irradiation times. Finally, when samples with a NMB concentration of 1000μM were irradiated, a complete fungicidal effect was achieved for all experimental combinations.
Therefore, on the basis of the results obtained by applying aPDT to cell suspensions of T. mentagrophytes (106 CFU/mL), we can deduce that with an agitation and irradiation time of 10minutes (9J/cm−2) the lowest concentration of NMB that achieves total cell mortality is 100μM; however, if the contact time remains the same but the sample is irradiated for 90minutes (81J/cm−2), the concentration of NMB can be decreased to 50μM.
DiscussionVarious in vitro studies have explored the possibility of PDT as treatment for dermatophytoses, in most cases using 5-aminolevulinic acid or derivatives thereof as the PS.8 In the case of onychomycosis in particular, Trichophyton rubrum has been the most-studied dermatophyte. Various scientific publications have shown that T. rubrum can be treated in vitro with PDT to obtain a fungicidal effect with various PSs, including toluidine blue O,9 5,10,15-tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride (Sylsens B),10–14 deuteroporphyrin monomethylester,10–12 5-aminolevulinic acid,14,15 and 1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[1,10,9,8-opqra]perylene-7,14-dione (hypericin).16
However, there have been few studies on the use of aPDT to treat fungal infections caused by T. mentagrophytes. In 2010, Jenefar et al.17 showed that this dermatophyte can be treated with aPDT using acriflavin at a concentration of 0.2μM, although it should be noted that the authors did not specify the dose of light used. Paz-Cristobal et al.16 successfully inactivated T. mentagrophytes using hypericin at a concentration of 20-50μM using different doses of light. It is difficult to compare our results with those of these authors because of the very different PSs and experimental conditions used. Dyes from the phenothiazinium family—including NMB—have also been used against T. mentagrophytes.18
Phenothiazinium dyes have also been found to be useful in PDT against various types of bacteria, including gram-positive Staphylococcus aureus and gram-negative Escherichia coli.19,20 NMB, in particular, has been found to be more effective than methylene blue, toluidine blue O, and dimethylmethylene blue against antibiotic-resistant strains of Acinetobacter baumannii.21 However, the concentration of NMB required to produce a bactericidal effect on Acinetobacter baumannii (2μM with a light dose of 30J/cm−2) is much lower than that required to inactivate CECT 2956T. mentagrophytes, according to our results in this study (50 vs 100μM at 81 and 9J/cm−2, respectively).
Larger cells are known to be less sensitive to aPDT than smaller cells. As a result, eukaryotes are more resistant to photoinactivation than bacteria because they have more targets per cell.22 Furthermore, in PDT it is generally accepted that the type ii mechanism yielding singlet oxygen (1O2) is the main pathway that causes cell damage. 1O2 is a ROS that does not interconvert with other ROSs, so its main characteristic, in relation to its photodynamic effect, is the length of its lifetime before it returns to its ground state by transferring its energy. The value of this parameter in the cellular environment where 1O2 can react with different biomolecules is unclear. In any case, because of its short useful lifetime, the distance 1O2 can travel from the site of its generation is limited to an estimated 270nm.23 This is a very short distance, even at the cellular scale. A typical prokaryote is only a few micrometers long, whereas eukaryotes often reach diameters of 10-30μm. Specifically, macroconidia of T. mentagrophytes that develop in a host can reach a length of 20-50μm and a width of 6-8μm. Consequently, the primary reactions of 1O2 in a cell take place within a short distance of where the molecule is generated. Therefore, at the molecular level, the place where 1O2 is generated is very important. The primary reactions of 1O2 with neighboring cells produce secondary ROSs capable of spreading and causing greater oxidative damage in cells.24
In the study by Rodrigues et al.,18 the treatment of T. mentagrophytes ATCC 9533 with NMB at a concentration of 10μM, an incubation time of 30minutes, and a light dose of 20J/cm−2 was sufficient to ensure that no surviving dermatophytes developed. In our study, a dose of 10μM was not sufficient to eradicate the CECT 2956 (ATCC 28443) strain in any case. A comparison of these 2 outcomes suggests that susceptibility to aPDT is strain-dependent because in both cases the incubation time of the suspensions—which underwent similar phototreatment, seeding, and incubation on SA—was 14 days.
Finally, because this study was carried out in vitro, its results cannot be directly extrapolated for the treatment of fungal infections in patients. Because of experimental difficulties, we were unable to study the photodynamic effects on the hyphae or macroconidia of T. mentagrophytes because laboratory-grown dermatophytes rarely generate these structures. Nevertheless, our results show that NMB is a PS that should be considered in the treatment of cutaneous fungal infections caused by this dermatophyte.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentialityThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
FundingThis study received aid from the Spanish Ministry of Economy and Competitiveness (CTQ2010-20870-C03-01 and CTQ2013-48767-C3-1-R).
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors are grateful to Sorisa for providing a Photocare lamp for this study.
Please cite this article as: López-Chicón P, Gulías Ò, Nonell S, Agut M. Terapia fotodinámica antimicrobiana in vitro aplicada sobre Trichophyton mentagrophytes con nuevo azul de metileno como fotosensibilizador. Actas Dermosifiliogr. 2016;107:765–770.