Clear cell sarcoma (CCS) is an uncommon malignant soft tissue tumor with cellular phenotypic characteristics similar to those of melanoma, thus necessitating a differential diagnosis, especially in metastatic melanocytic lesions of unknown primary site.1
Perivascular epithelial cell tumors (PEComas), on the other hand, are mesenchymal neoplasms composed of epithelioid cells that rarely affect the skin and whose immunohistochemical characteristics are similar to those of CCS.2
We report the case of a 45-year-old woman who underwent treatment for pain and paresthesia affecting the territory of the median nerve of the right hand. These findings were compatible with carpal tunnel syndrome and were confirmed using electromyography.
Carpal tunnel release was performed, as was a histopathology workup of the ligament.
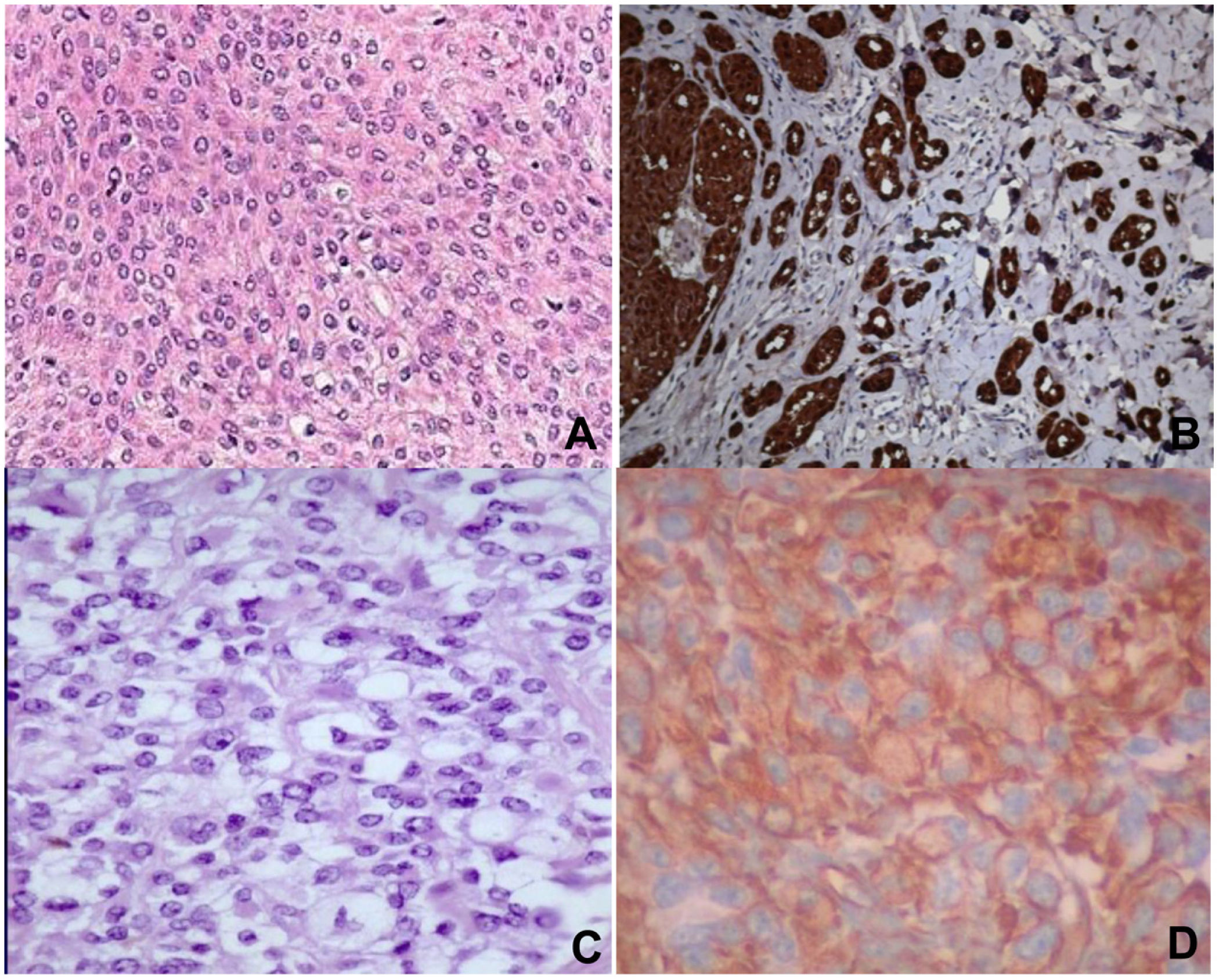
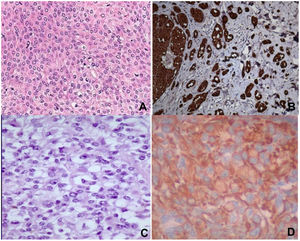
Histopathology revealed spindle cell proliferation comprising irregularly arranged cells that infiltrated the conjunctive tissue and nerve structures with 10 mitotic figures per 10 high-power fields (HPFs). Immunostaining was positive for HMB-45, S-100, and vimentin, focally positive for collagen IV and factor XIIIA, and negative for muscle-specific actin, cytokeratin, neuron-specific enolase, CD34, and CD68 (Fig. 1A and B).
A) Staining with hematoxylin–eosin showing clear cells, epithelioid cells, and spindle cells. B) Spindle cells expressing S-100. C) Staining with hematoxylin-eosin showing spindle cells with a clear cytoplasm and minimal cytologic atypia. D) Cells expressing vimentin. Original magnification for all images, 40×.
The identification of the genetic rearrangement of EWS-ATF1 confirmed the chromosomal translocation t(12;22)(q13;q12), thus corroborating the diagnosis of CCS. Selective sentinel lymph node biopsy revealed no evidence of malignant disease, and adjuvant treatment with dacarbazine was administered. The disease metastasized to the bones of both shoulders and multiple vertebrae; therefore, the patient received a second cycle of chemotherapy with a platinum-based drug and etoposide and radiotherapy, although these were not effective. The disease spread to the lungs, and the patient was sent to palliative care. She died 12 months after surgery.
Another case involved a 32-year-old woman referred with a blackish-blue nodule in the left lumbar area that was ulcerative in appearance and had doubled in size in 2 months. She also had multiple enlarged lymph nodes in the left groin. Biopsy was suggestive of melanoma. On suspicion of metastatic melanoma, the surgical margins were extended, and left inguinal lymphadenectomy was performed owing to the suspicion of locoregional lymph node metastasis.
Histopathology revealed infiltration of the skin and soft tissue by a malignant tumor composed of spindle cells and perivascular epithelioid cells, with metastases in 2 of 8 lymph nodes. Immunostaining was positive for HMB-45, melan-A, and vimentin, focally positive for S-100 and CD68, and negative for actin, α-1-antitrypsin, CD117, CD34, CD99, CKAE1/AE13, p53, desmin, and synaptophysin (Fig. 1C and D).
These findings led to a differential diagnosis between CCS and PEComa. Given that the negative result for the rearrangement EWS-ATF1 ruled out CSS, the patient was eventually diagnosed with PEComa.
Treatment consisted of 4 cycles of chemotherapy (ifosfamide/doxorubicin). Follow-up computed tomography revealed that the tumor had progressed to the lungs. Palliative chemotherapy was started with dacarbazine in monotherapy. Sunitinib 50 was administered after further progression of the tumor. Nevertheless, the number of lung nodules increased, as did that of the pericardial, retroperitoneal, and mediastinal metastatic nodules, leading to a gradual deterioration of the patient's general status. The patient died 11 months after surgery.
CCS is a clinical and pathological entity that frequently affects the soft tissues in the limbs of young adults, mainly adjacent to tendons and aponeuroses.1 It accounts for 1% of soft tissue sarcomas.1
The prognosis of CCS is poor, with a high percentage of recurrence (21%-69%),3 5-year survival of 47%–67%, and 10-year survival of 33%-36%.3 The indicators of poor prognosis are tumor size >5cm, presence of tumor necrosis, complete surgical resection, high mitotic index (>2 mitotic figures per 10 HPFs), depth, TNM classification, presence of metastasis, and local recurrence.3,4
The differential diagnosis of CCS includes both epithelial and mesenchymal tumors, although it is extremely difficult to make the diagnosis with metastasis of an occult melanoma and with PEComa.5 Confirmation of the disease prevents incorrect treatment and enables prognosis to be determined (Tables 1 and 2).
Differential Diagnosis of Clear Cell Sarcoma with Melanoma and PEComa According to Immunohistochemical Characteristics and Chromosomal Rearrangements.
| Clear cell sarcoma | Melanoma | PEComa | |
|---|---|---|---|
| S100 | + | + | ± |
| HMB-45 | + | + | + |
| Melan-A | + | + | ± |
| MITF | + | + | + |
| Smooth muscle actin | (+occasionally focal) | − | ± |
| Epithelial membrane antigen | + | − | ± |
| Desmin | (+occasionally focal) | − | ± |
| Vimentin | + | + | + |
| T(12;22)(q13;q12) | + | − | − |
| BRAF mutation | − | + | − |
| CD10 | − | + | + (cutaneous) |
Compared with melanoma, CCS is characterized by spindle cell growth or pale, clear cell nests with phenotypic characteristics similar to those of malignant melanoma and including the presence of melanin, melanosomes, immunostaining of S-100, and markers of melanoma, such as HMB-45, melan-A, and MITF,1 as in the first case we report.
The genetic rearrangement t(12;22)(q13;q12), which enables the fusion of EWS and ATF1, is the chromosomal abnormality that made it possible to differentiate between CCS and melanoma.1,5 CCS in the gastrointestinal tract is characterized by a variant in the EWSR1-CREB1 fusion,1,5 whereas the most frequent genetic abnormalities in melanoma are mutations in the BRAF gene (mainly V600) and in NRAS.6.
PEComa is a mesenchymal tumor formed by perivascular epithelioid cells, which can even infiltrate smooth muscle. It expresses melanocytic markers and its behavioral spectrum ranges from benign to frankly malignant,2 as in the second case we report. Its phenotype is histologically and immunohistochemically similar to CCS, although cutaneous PEComa usually expresses CD10, which is very useful in the differential diagnosis.7 Negative results in immunostaining for smooth muscle actin and desmin make it possible to distinguish between these entities and to identify the translocation t(12;22)(q13;q12),2 as in the second patient, where it proved useful for ruling out the diagnosis of CCS. Given that some genetic abnormalities have been reported to affect activation of the mTOR complex, the disease can be treated with the mTOR complex inhibitor sirolimus.8
Treatment of CCS involves radical excision of the tumor. There are no data to justify generalized adjuvant therapy.9
Radiotherapy is used in patients with positive margins.10 Chemotherapy could prove beneficial in patients with metastatic disease.10
Conflicts of InterestThe authors declare that they have no conflicts of interest.