Vitiligo is a chronic autoimmune skin disease caused by the destruction of melanocytes. Although quality of life (QOL) in vitiligo has been studied in different countries, it has not yet been investigated in Mexico. The aim of this study was to assess the QOL of Mexican patients with vitiligo.
Material and methodWe conducted a cross-sectional study at the research unit of Centro Dermatológico Dr. Ladislao de la Pascua in Mexico City. We included adults with vitiligo and excluded those with other pigmentation disorders or a neurological or psychiatric disorder. Patients on psychoactive medications were also excluded. All the patients were administered the Dermatology Life Quality Index (DLQI), a vitiligo-specific quality of life instrument (the VitiQoL), and the Beck Depression and Anxiety Inventories.
ResultsWe studied 150 patients with vitiligo (103 women [68.7%] and 47 men [31.3%]). The median (interquartile range) age was 38 (20) years. The mean (SD) scores on the DLQI and VitiQoL were 5.2 (5.4) and 32.1 (22.7) out of total possible scores of 30 and 90, respectively. The correlation between questionnaire scores was 0.675 (P<.001). Patients with genital involvement scored significantly worse on the VitiQoL than those without lesions in this area (43.95 [28.4]) vs 28.98 [20.08], P<.001). The prevalence of depression and anxiety was 34% and 60%, respectively.
ConclusionVitiligo has a minimal impact on the QOL of our patients. QOL was worse in patients with genital lesions.
El vitíligo es una dermatosis autoinmune crónica causada por la destrucción de los melanocitos. Aunque la calidad de vida en pacientes con vitíligo se ha estudiado en diferentes poblaciones, hasta el momento no existen estudios previos sobre el tema en nuestra población. El objetivo de este estudio era determinar la calidad de vida de pacientes con vitíligo mexicanos.
Material y métodoSe realizó un estudio transversal en la Unidad de Investigación del Centro Dermatológico Dr. Ladislao de la Pascua en la ciudad de México. Se reclutaron adultos con vitíligo, se excluyeron los individuos con otras alteraciones de la pigmentación, diagnóstico de enfermedad neurológica y/o psiquiátrica y en tratamiento farmacológico con sustancias que afectaran su estado mental. Todos los pacientes contestaron los cuestionarios DLQI y VitiQoL y los inventarios de depresión y ansiedad de Beck.
ResultadosSe reclutaron 150 pacientes con vitíligo, 68,7% (103) mujeres y 31,3% (47) hombres. La mediana de edad fue de 38 años±20 años. En nuestros pacientes el promedio de la puntuación del DLQI fue de 5,2±5,4 y el del VitiQoL fue de 32,1 (DE: 22,7), de los 30 y 90 puntos posibles de cada instrumento, respectivamente. La correlación de los resultados de ambos cuestionarios fue de 0,675, p<0,001. Los pacientes con afectación de genitales reportaron una puntuación mayor en el cuestionario de calidad de vida VitiQoL que aquellos sin afectación de esa zona corporal, 43,5 (DE: 28,4) vs 28,98 (DE: 20,08), p<0,001. La prevalencia de depresión y ansiedad fue del 34% y 60%, respectivamente.
ConclusiónEl impacto del vitíligo en la calidad de vida de nuestra muestra de pacientes fue mínimo. El vitíligo en los genitales se asocia a un deterioro de la calidad de vida.
Vitiligo is a chronic autoimmune skin disease characterized by white or hypopigmented macules.1–3 The reported prevalence ranges from 0.06% to 2.28% around the world.4 Forms of the disease are classified as segmental, nonsegmental or indeterminate (unclassifiable).5 Topical or systemic corticosteroids are the first line of therapy.6
Quality of life in dermatology refers to the patient's view of changes brought about by a skin disease.7 The most widely used instrument to measure the patient's perception is the Dermatology Life Quality Index (DLQI).8 A disease-specific measure, however, is provided by the Vitiligo Quality of Life (VitiQOL) index.9 Both tools are positively correlated (r=0.83).9
Vitiligo has been linked to psychiatric diseases such as depression, experienced by 59% of patients with this skin disease.10 One screening tool available is the Hamilton Rating Scale for Depression; others that are often administered in clinical settings are the Montgomery-Asberg Depression Rating Scale and the Beck Depression Inventory.11–13 The construct validity of the Beck scale also provides an instrument useful for detecting anxiety.14
Although quality of life in vitiligo has been studied in various populations, it has not been examined in our practice setting of Mexico City. Our aim was to measure the quality of life of adults diagnosed with vitiligo in our referral hospital for skin diseases.
Patients and MethodsThis cross-sectional study was undertaken in the Dr Ladislao de la Pascua Dermatology Research Center in Mexico City from October 2014 to June 2015. The study was approved by the center's ethics committee and the participants gave their signed informed consent. Patients with vitiligo who were over the age of 18 years were included. We excluded patients with other skin diseases affecting pigmentation and those with neurological diseases.
A single dermatologist took each participant's medical history and recorded the following data: sociodemographic characteristics, clinical type, percentage of body surface affected, time since onset, family history of vitiligo, prior treatments, signs and symptoms, and autoimmune diseases. The patients completed the Spanish versions of the DLQI and VitiQOL questionnaires. The research team undertook to produce a culturally adapted translation of the VitiQOL following the recommendations of the International Society for Pharmacoeconomics and Outcomes Research.15 We also administered the depression and anxiety sections of the Beck Depression Inventory to screen for psychiatric disease.
Data were analyzed with SPSS software (version 19). Qualitative variables were described with percentages. Normally distributed quantitative variables were described with the mean (SD) and nonnormally distributed results with percentiles. Statistical significance was set at a value of P<.05. We calculated the sample size required for a power of 0.96 (α error, 0.05) using the G*Power software, version 3.1.
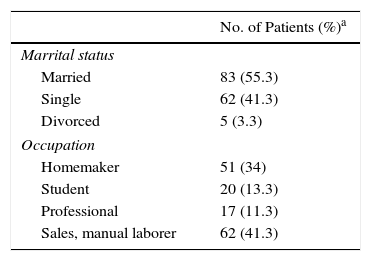
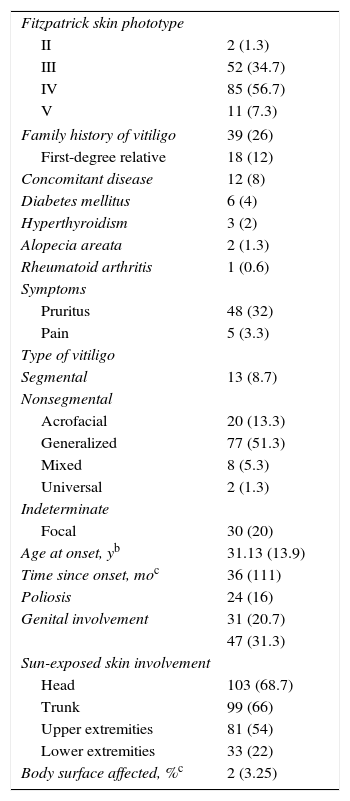
ResultsWe recruited 150 patients with vitiligo: 103 (68.7%) were women and 47 (31.3%) were men. The mean age was 38 (20) years. Other characteristics are shown in Tables 1 and 2. Most patients were being treated with corticosteroids, calcineurin inhibitors, and topical psoralen.
Clinical Characteristics of the Cohorta
| Fitzpatrick skin phototype | |
| II | 2 (1.3) |
| III | 52 (34.7) |
| IV | 85 (56.7) |
| V | 11 (7.3) |
| Family history of vitiligo | 39 (26) |
| First-degree relative | 18 (12) |
| Concomitant disease | 12 (8) |
| Diabetes mellitus | 6 (4) |
| Hyperthyroidism | 3 (2) |
| Alopecia areata | 2 (1.3) |
| Rheumatoid arthritis | 1 (0.6) |
| Symptoms | |
| Pruritus | 48 (32) |
| Pain | 5 (3.3) |
| Type of vitiligo | |
| Segmental | 13 (8.7) |
| Nonsegmental | |
| Acrofacial | 20 (13.3) |
| Generalized | 77 (51.3) |
| Mixed | 8 (5.3) |
| Universal | 2 (1.3) |
| Indeterminate | |
| Focal | 30 (20) |
| Age at onset, yb | 31.13 (13.9) |
| Time since onset, moc | 36 (111) |
| Poliosis | 24 (16) |
| Genital involvement | 31 (20.7) |
| 47 (31.3) | |
| Sun-exposed skin involvement | |
| Head | 103 (68.7) |
| Trunk | 99 (66) |
| Upper extremities | 81 (54) |
| Lower extremities | 33 (22) |
| Body surface affected, %c | 2 (3.25) |
The mean DLQI was 5.2 (5.4) out of a total of 30 possible points. The mean VitiQOL index was 32.1 (22.7) out of a total of 90 points. The correlation between the 2 indexes was 0.675 (P<.001). DLQI and VitiQOL question results are detailed in Tables 3 and 4, respectively. Question 7 of the DLQI received the lowest ratings: 91.3% of the patients felt that vitiligo had no impact on their work. On none of the questions did the mean responses approach the maximum score of 3, which would have indicated the patient was greatly affected. The mean overall scores for men and women were similar (men, 4.8 [4.37]; women, 3.4 [4.01], P=.075). The means for married patients (5.02 [4.75]) tended to be higher than those of unmarried patients (3.88 [3.98]) but the difference was not significant (P=.176). Patients with fewer than 12 years of formal education had scores that were similar to others with more formal schooling (3.29 [2.73] vs 4.25 [4.58], respectively; P=.441). The results showed no correlation between time since onset and the DLQI (r=–0.015, P=.861) or between the DLQI and the percentage of body surface affected (r=.154, P=.072).
DLQI Results, by Question
| Questions Over the last week: | Mean (SD)a |
|---|---|
| 1. How itchy, sore, painful or stinging has your skin been? | 0.69 (0.8) |
| 2. How embarrassed or self-conscious have you been because of your skin? | 0.81 (0.9) |
| 3. How much has your skin interfered with going shopping or looking after your home or garden? | 0.49 (0.8) |
| 4. How much has your skin influenced the clothes you wear? | 0.74 (1.3) |
| 5. How much has your skin affected any social or leisure activities? | 0.55 (0.8) |
| 6. How much has your skin made it difficult for you to do any sport? | 0.55 (0.9) |
| 7. How much has your skin prevented you from working or studying? | 0.13 (0.5) |
| Or if not, how much has your skin been a problem at work or studying? | 0.14 (0.3) |
| 8. How much has your skin created problems with your partner or any of your close friends or relatives? | 0.34 (0.7) |
| 9. How much has your skin caused any sexual difficulties? | 0.37 (0.8) |
| 10. How much of a problem has the skin treatment been? For example, by making your home messy or by taking up time. | 0.38 (0.8) |
VitiQOL Results, by Question
| Questions During the last month: | Mean (SD) a |
|---|---|
| 1. Did you feel bothered by the appearance of your skin? | 3.2 (2.2) |
| 2. Did you feel frustrated about your skin condition? | 2.8 (2.2) |
| 3. Did you feel difficulty in showing affection because of your skin condition? | 1.4 (2.1) |
| 4. Has your skin condition affected your daily activities? | 0.97 (1.7) |
| 5. While talking to someone, did you worry about what people might be thinking about you? | 2.1 (2.3) |
| 6. Were you afraid that people would criticize you? | 2.4 (2.4) |
| 7. Did you feel embarrassed or inhibited because of your skin? | 2.4 (2.2) |
| 8. Did the appearance of your skin influence your choice of clothing? | 1.7 (2.3) |
| 9. Did your skin condition affect your social or leisure activities? | 1.4 (1.9) |
| 10. Did your skin condition affect your emotional well-being? | 2.4 (2.2) |
| 11. Did your skin condition affect your physical health (as a whole)? | 11.2 (1.9) |
| 12. Did your skin condition influence your care of personal appearance (for instance, haircut or use of cosmetic products)? | 1.5 (2.2) |
| 13. Did your skin condition influence your sun protection care during leisure activities (for instance, limiting the time of exposure to the sun during peak hours, staying out of the sun, wearing a hat, long sleeves or pants)? | 3.9 (2.2) |
| 14. Did your skin condition affect the possibility of making new friends? | 0.74 (1.6) |
| 15. Did you worry about disease progression to other body parts? | 4.2 (2.1) |
| 16. Please rate how severe you think your skin condition is on the scale. | 2.7 (1.7) |
Questions 1 (feeling uncomfortable about appearance because of vitiligo) and 13 (changes in sun-related skin care) on the VitiQOL received the highest ratings, but they did not approach the maximum score of 6. Questions 4 (activities of daily living) and 14 (making new friends) received the lowest ratings. Similar results were recorded for men and women (31.6 [21.17] and 36.2 [24.62], respectively; P=.272). The scores of married and unmarried patients were also similar (35.35 [24.75] and 35.19 [23.26], respectively; P=.971). Likewise, scores did not differ in relation to whether patients had less than 12 years of formal education (36.53 [25.26]) or 12 or more years (35.63 [22.31]) (P=.897), and there was no correlation between time since onset of vitiligo and VitiQOL score (r=0.227, P=.005) or score and percentage of body surface affected (r=0.349, P=.0001).
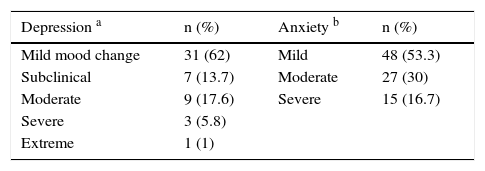
Beck Inventory results showed that 34% of patients had some degree of depression and 60% had some degree of anxiety. Results are detailed in Table 5. Quality of life showed a moderate correlation with depression (r=0.598; P<.05) and anxiety (r=0.424; P<.05). DLQI and VitiQOL results did not differ in relation to the following variables: sex, age, marital status, occupation, body surface affected, time since onset, skin phototype, or sun exposure. Patients with genital involvement reported higher VitiQOL ratings than those without such involvement (43.95 [28.4] vs 28.98 [20.08], respectively; P<.001). However, the DLQI results for these 2 groups were similar (4.4 [4.8] vs 4.3 [4.1]; P=.96). Results were also similar for patients with universal vitiligo and focal vitiligo, where differences were only seen in VitiQOL scores (universal, 87 [4.24); focal, 24.55 [16.7]; P<.001).
DiscussionIn contrast with previous reports about the impact of vitiligo on quality of life, we found that the effect was slight in our study cohort, probably in relation to the extension of affected areas and shorter times since onset in our patients. Their mean DLQI score of 5.2 was lower than the score of 8.2 reported in the review by Amer andGao16 but similar to results reported by Ongenae et al.17 (4.95) for Belgium, Tanioka et al.18 (5.9) for Japan, or Silverberg and Silverberg19 (5.9) for the United States. Our Mexico City patients’ mean score of 5.2 points on a scale of 0 to 30 points reflects only a slight impact of vitiligo compared to scores in Saudi Arabia20 and Turkey,21 where mean scores of 14.72 and 15 points, respectively, were reported. The differences can be attributed to sociocultural issues and myths prevalent in those countries. Fatanietal.22 found that 24.1% of persons in Tanzania would avoid shaking hands with someone with vitiligo for fear of contagion, 24.8% would not sit to eat at the same table, and 42.8% would not marry someone with the disease.
Women have been found to experience greater impact on quality of life than men (scores of 8.03 vs 5.99, respectively16). Likewise, married patients are more negatively affected than unmarried ones (scores of 9.22 vs 6.91, respectively23). A lower educational level, on the other hand, has been reported to be associated with less impact on quality of life.23 However, we detected none of these sociodemographic differences, which might be attributable to selection bias.10 Nor did we detect a significant correlation between quality of life and disease extension or the presence of patches on sun-exposed skin, probably because half our sample reported that disease onset had occurred less than 3 years earlier. In contrast other researchers studied patients who had lived with the disease for much longer periods.19,24 Our findings might also be attributable to a low percentage of body surface area affected (2%). Kiprono et al.24 and Karelson et al.25 found that differences in quality of life began after disease extension reached 9.15% and 10% of the skin surface.
Hedayat et al.26 reported that psychiatric disorders such as depression and anxiety do not affect quality of life in vitiligo, but we found that 34% of our patients had some degree of depression according to the Beck Inventory,12 consistent with reports for Hindi-speaking populations in India with depression prevalences of 23.3% and 59%.10,28,29 Similarly, psychiatric disorders have been reported in 20.3% to 29.3% of patients with vitiligo in other studies in India, where the disease is stigmatized.10,27 Sixty percent of our sample presented some degree of anxiety, a much higher prevalence than the 3.3% reported by Sangma et al.10 We must point out that the presence of psychiatric disorders may be unrelated to vitiligo, however, given that no psychiatric interviews were conducted to explore possible causes of depression and anxiety.
Our approach to patient recruitment, which did not employ random sampling to support representativeness, places limitations on our conclusions. However, the percentages of clinical forms of vitiligo in our sample reflect the range reported in the literature. Another limitation is that the data we present for depression and anxiety were based on screening instruments, which cannot substitute for psychiatric evaluations.
We conclude that quality of life in patients with vitiligo should be assessed with screening questionnaires to detect psychiatric disorders in the interest of treating the whole patient. It remains to establish whether psychiatric disorders are secondary to the disease or merely concomitant conditions.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed adhered to the ethical guidelines of the responsible committee on human experimentation and comply with the Declaration of Helsinki of the World Medical Association.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morales-Sánchez MA, Vargas-Salinas M, Peralta-Pedrero ML, Olguín-García MG, Jurado-Santa Cruz F. Impacto del vitíligo en la calidad de vida. Actas Dermosifiliogr. 2017;108:637–642.