The development of drugs capable of stimulating the body's immune response to destroy tumor cells has revolutionized the treatment of advanced melanoma. Ipilimumab, a-cytoxic T lymphocyte antigen (CTLA-4) blocking antibody with approval for the treatment of metastatic melanoma and as an adjuvant in stage iii melanoma, was the first of a growing list of immunotherapy agents (Table 1) to offer increased survival in this setting.1 Compared with anti-CTLA-4 antibodies, pembrolizumab and nivolumab, 2 anti-programmed death 1 (PD1) antibodies, are associated with increased survival in patients with metastatic melanoma, and are considered a first-line treatment for patients with wild-type BRAF melanoma and part of the first-line treatment for those with BRAF-mutated melanoma. The combined use of these drugs with anti-CTLA-4 antibody therapy boosts response but also increases toxicity.1 Their role as adjuvants in the treatment of melanoma is currently being investigated in several open-label clinical trials.
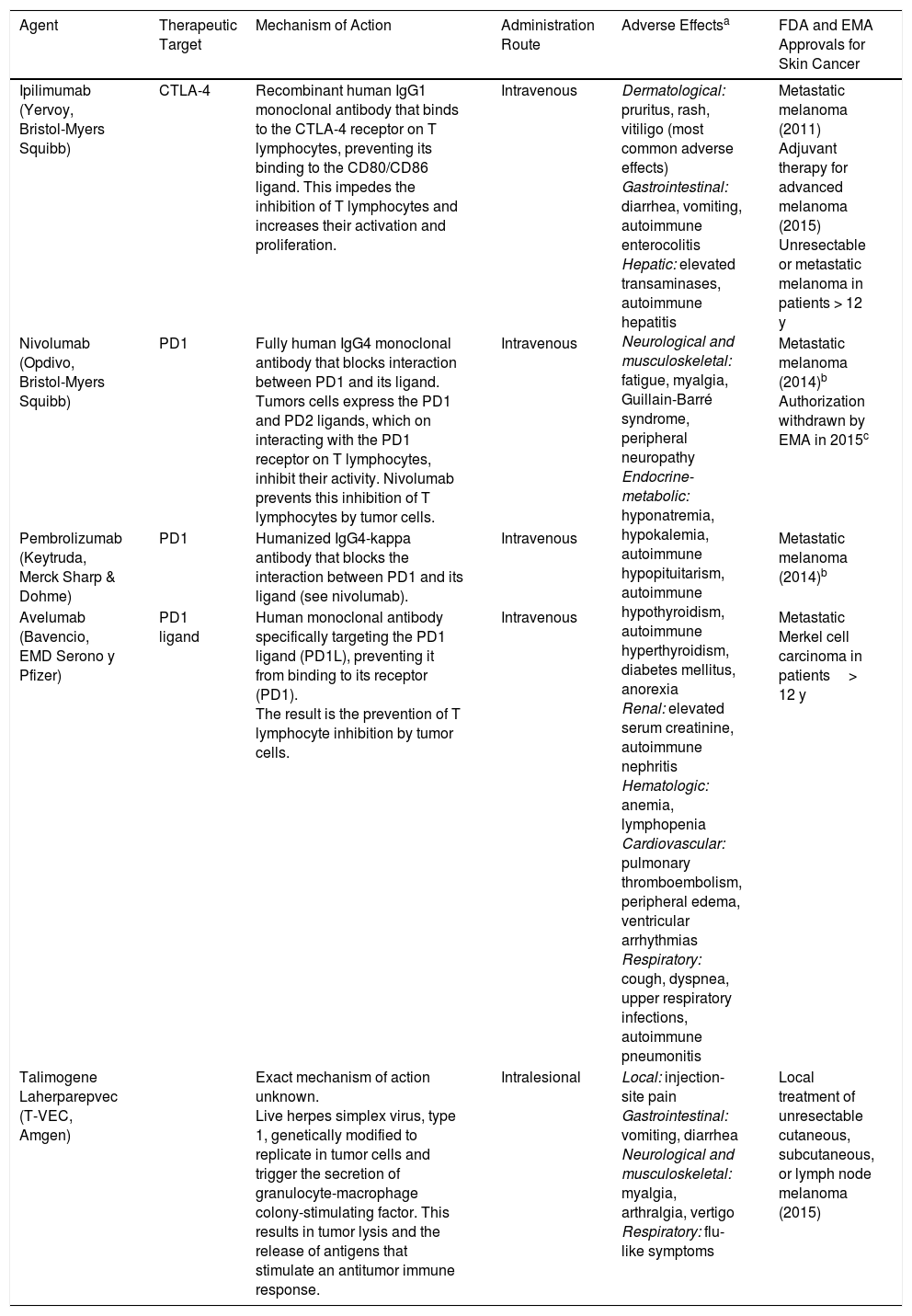
New Immunotherapy Agents Used in Advanced Skin Cancer.
| Agent | Therapeutic Target | Mechanism of Action | Administration Route | Adverse Effectsa | FDA and EMA Approvals for Skin Cancer |
|---|---|---|---|---|---|
| Ipilimumab (Yervoy, Bristol-Myers Squibb) | CTLA-4 | Recombinant human IgG1 monoclonal antibody that binds to the CTLA-4 receptor on T lymphocytes, preventing its binding to the CD80/CD86 ligand. This impedes the inhibition of T lymphocytes and increases their activation and proliferation. | Intravenous | Dermatological: pruritus, rash, vitiligo (most common adverse effects) Gastrointestinal: diarrhea, vomiting, autoimmune enterocolitis Hepatic: elevated transaminases, autoimmune hepatitis Neurological and musculoskeletal: fatigue, myalgia, Guillain-Barré syndrome, peripheral neuropathy Endocrine-metabolic: hyponatremia, hypokalemia, autoimmune hypopituitarism, autoimmune hypothyroidism, autoimmune hyperthyroidism, diabetes mellitus, anorexia Renal: elevated serum creatinine, autoimmune nephritis Hematologic: anemia, lymphopenia Cardiovascular: pulmonary thromboembolism, peripheral edema, ventricular arrhythmias Respiratory: cough, dyspnea, upper respiratory infections, autoimmune pneumonitis | Metastatic melanoma (2011) Adjuvant therapy for advanced melanoma (2015) Unresectable or metastatic melanoma in patients > 12 y |
| Nivolumab (Opdivo, Bristol-Myers Squibb) | PD1 | Fully human IgG4 monoclonal antibody that blocks interaction between PD1 and its ligand. Tumors cells express the PD1 and PD2 ligands, which on interacting with the PD1 receptor on T lymphocytes, inhibit their activity. Nivolumab prevents this inhibition of T lymphocytes by tumor cells. | Intravenous | Metastatic melanoma (2014)b Authorization withdrawn by EMA in 2015c | |
| Pembrolizumab (Keytruda, Merck Sharp & Dohme) | PD1 | Humanized IgG4-kappa antibody that blocks the interaction between PD1 and its ligand (see nivolumab). | Intravenous | Metastatic melanoma (2014)b | |
| Avelumab (Bavencio, EMD Serono y Pfizer) | PD1 ligand | Human monoclonal antibody specifically targeting the PD1 ligand (PD1L), preventing it from binding to its receptor (PD1). The result is the prevention of T lymphocyte inhibition by tumor cells. | Intravenous | Metastatic Merkel cell carcinoma in patients> 12 y | |
| Talimogene Laherparepvec (T-VEC, Amgen) | Exact mechanism of action unknown. Live herpes simplex virus, type 1, genetically modified to replicate in tumor cells and trigger the secretion of granulocyte-macrophage colony-stimulating factor. This results in tumor lysis and the release of antigens that stimulate an antitumor immune response. | Intralesional | Local: injection-site pain Gastrointestinal: vomiting, diarrhea Neurological and musculoskeletal: myalgia, arthralgia, vertigo Respiratory: flu-like symptoms | Local treatment of unresectable cutaneous, subcutaneous, or lymph node melanoma (2015) |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte antigen 4; PD1, programmed death 1; EMA, European Medicines Agency; FDA, US Food and Drug Administration.
Early detection and treatment of adverse effects is important. These can be treated with corticosteroids and immunosuppressive drugs (infliximab, mycophenolate). Depending on the severity of the effects, withdrawal of the drug may be necessary.
The combined use of ipilimumab and nivolumab is associated with a considerable increase in toxicity (98% of patients had adverse effects and 53% of these were grade 3-4).1
Talimogene laherparepvec (T-VEC) is an oncolytic virus administered intralesionally with approval from the US Food and Drug Administration (FDA) for the treatment of unresectable metastatic melanoma.1 Its usefulness when combined with anti-PD1 antibodies is also being studied in clinical trials.
Although immunotherapy has earned itself a place in the treatment of melanoma, its benefits in other skin cancers remain to be demonstrated.
Metastatic Merkel cell carcinoma is difficult to treat and response to chemotherapy is short-lived. Avelumab is a monoclonal anti-PDL1 antibody that was recently approved by the FDA for the treatment of metastatic Merkel cell carcinoma. The approval was based on the results of a phase II clinical trial, JAVELIN-Merkel-200, which involved 88 patients, most of whom had a primary cutaneous tumor. The trial showed an overall response rate of 31.8% (n=28) (95.9% CI, 21.9-43.1), which included 8 complete responses. At the time of data analysis, the mean duration of response was in excess of 10.4 months. Five of the 88 patients (6%) developed serious adverse effects.2
The treatment options for unresectable basal cell carcinoma are limited, and there are currently no alternatives for patients who do not respond to Hedgehog inhibitors (vismodegib, sonidegib, saridegib). PDL1 expression, however, has been reported in neoplastic cells and intratumoral lymphocytes in basal cell carcinoma.3 In addition, complete clinical response to pembrolizumab has been described in a patient with metastatic basal cell carcinoma that had progressed despite treatment with saridegib.3
There is no consensus on the management of unresectable or metastatic squamous cell carcinoma (SCC). One recent study reported increased expression of PD1 and its ligands in 38 biopsy samples from 24 patients with SCC, mostly high-risk. The expression was particularly evident in tumors with perineural invasion.4 There has also been a report of a patient with locally advanced SCC who experienced complete remission with pembrolizumab.4 The preliminary results of a phase I trial investigating the use of REGN2810, an anti-PD1 antibody, in patients with advanced SCC were presented at the 2017 meeting of the American Society of Clinical Oncology and showed an overall response rate of 52% (12/23). Two of the patients achieved complete response and the treatment was well tolerated.5
The use of immunotherapy to combat skin cancer is set to grow exponentially in coming years. Dermatologists need to be familiar with these new drugs and their adverse effects in order to offer optimal management to patients with advanced skin cancer.
Please cite this article as: Morgado-Carrasco D, Terc F, Ertekin SS, Ferrandiz L. FR-Inmunoterapia en el cáncer cutáneo avanzado. Actas Dermosifiliogr. 2019;110:53–56.