Immunophenotypic shift during the progression of mycosis fungoides (MF) is a rare event, and its prognostic significance is unknown.
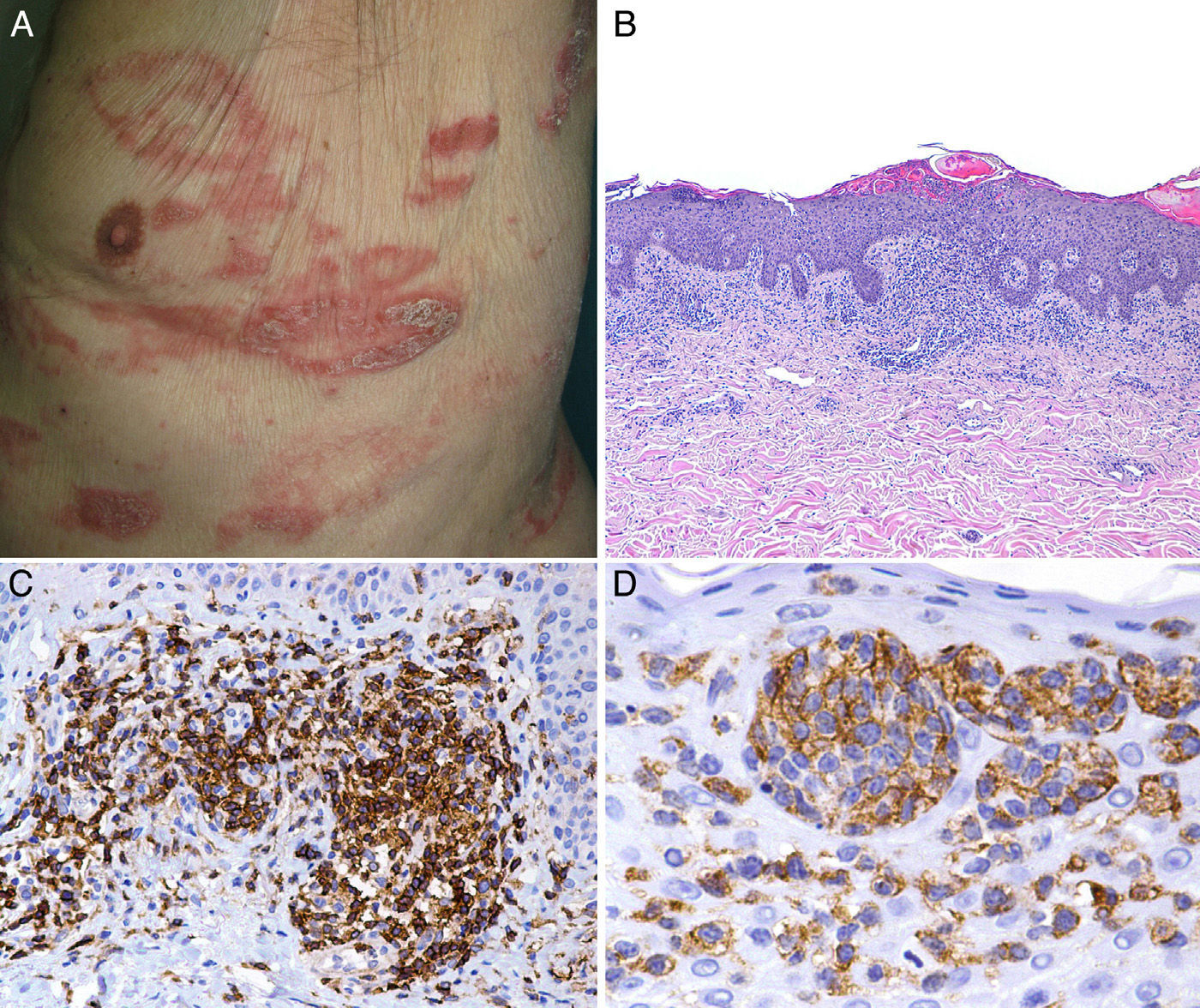
We present the case of a man aged 84 years who presented a 2-year history of generalized pruritus. Over the previous 5 months he had developed erythematous plaques of different sizes mainly on the trunk; the plaques had an annular or arcuate morphology and some showed desquamation (Fig. 1A).
A, Infiltrated, desquamating erythematous plaques with an arcuate morphology on the trunk. B, Dense infiltrate formed of atypical lymphocytes with epidermotropism. Hematoxylin and eosin, original magnification×20.C, Positivity for CD4. D, Positivity for CD30 in the epidermotropic infiltrate.
Biopsy of a plaque on the abdomen revealed a dermal infiltrate of atypical and pleomorphic (small, medium-sized and large) lymphocytes with hyperchromatic cerebriform nuclei, showing a tendency to epidermotropism, forming large intraepidermal aggregates (Fig. 1B). Large lymphocytes accounted for less than 25% of the cells of the infiltrate. Immunohistochemistry was positive for CD2, CD3, CD4, CD5, CD6, and CD7. The CD8 antigen was focally positive in the dermis, the CD4:CD8 ratio was 4:1, and CD30 was intensely expressed in the intraepidermal lymphocytes (Fig. 1, C and D). Polymerase chain reaction analysis detected T-cell receptor (TCR) γ-chain gene clonal rearrangement. No expression of CD56, TIA-1, or granzyme B was detected. There were no significant changes on other additional laboratory tests. Based on these findings, we made a diagnosis of MF-type primary cutaneous T-cell lymphoma, stageIB. Treatment was initiated with topical corticosteroids and psoralen–UV-A, with partial improvement of the skin lesions.
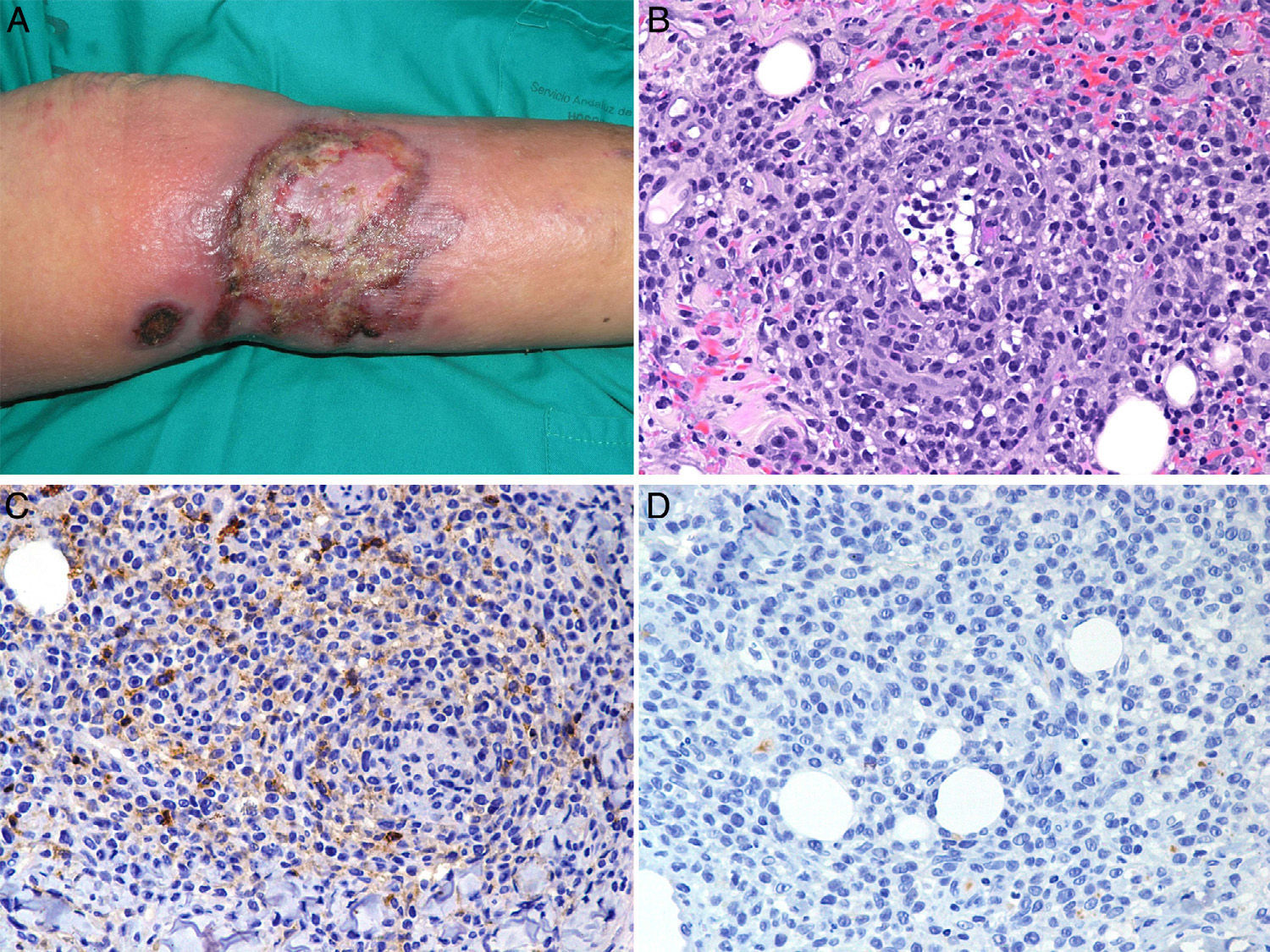
At 6 months the patient developed a large ulcerating necrotic tumor plaque with erythematous-violaceous borders on the medial surface of the left lower leg (Fig. 2A). Further biopsies were taken and a monomorphic infiltrate of large atypical lymphocytes was seen to affect the full thickness of the dermis with extension into the subcutaneous adipose tissue. There was a notable angiocentricity, with blood vessel necrosis and invasion of vessel walls by the atypical lymphocytes (Fig. 2B). Our differential diagnosis based on these data included progression of MF or the onset and a new lymphoproliferative process (extranodal NK/T-cell lymphoma, nasal type; γ/δ T-cell lymphoma, angioinvasive (type E) lymphomatoid papulosis,1 or B-cell lymphoma rich in angioinvasive T cells2). The new immunohistochemistry analysis was positive for CD3, CD5, CD7, and βF1, and showed expression of granzyme B and TIA-1. CD2, CD4, CD8, and CD30 expression was negative, as was CD56, CD20, EBER, and TCR-γ expression (Fig. 2, C and D). Clonality of the TCR-γ gene was once again demonstrated on PCR, with a clonal peak of identical size to that of the earlier lesions. The other additional tests, including computed tomography and bone-marrow biopsy were normal. Two months later the patient died as a result of rapidly progressive septicemia.
In the majority of cases of MF, immunophenotypic expression is CD3+, CD4+ and CD8−.3,4 Some cases show a cytotoxic/suppressor profile (CD3+, CD4−, CD8+)5,6 or other aberrant phenotypes. Immunophenotypic shift during disease progression is rare, with only 2 cases reported in the literature. In the first of those cases, 5 years after a diagnosis of CD4+/CD8− MF with lesions on the trunk, the patient presented cutaneous tumors associated with a loss of vision in the left eye. Histopathology of the globe showed infiltration of the vitreous by atypical CD8+ lymphocytes, with a CD4:CD8 ratio of 1:4. Although clonal rearrangement of the TCR gene was not mentioned, lesion progression to a tumor phase with concomitant involvement of the vitreous would suggest that the same lymphoproliferative process was the origin.7 In the second case, the diagnosis was also CD4+/CD8− MF. After a number of recurrences, the patient developed ulcerated lesions on the lower limbs; the immunohistochemical changes also consisted of a loss of CD4 expression and an increase in the number of CD8+ atypical lymphocytes.8 Clonal rearrangement of the TCR gene was detected, and comparison of the initial CD4+ lesions with the later CD8+ lesions showed the clonal peaks to be of identical size.
In our patient, neither CD4 nor CD8 expression was detected in the immunophenotypic study of the ulcerated tumor plaque, but the clonal peaks were of an identical size to those in the initial lesions; this therefore indicated that the lesions with distinct clinical and immunophenotypic characteristics were clonally related. To date, there are no reports of cases with loss of CD4 expression. The mechanism that produces the changes in immunophenotype is unknown. This phenomenon is considered common in the case of some childhood B- and T-cell leukemias; however, reports of such changes in the case of B-cell or T-cell lymphoma are rare in the literature.
We have presented the first case of MF with shift to a double-negative immunophenotype (CD4−/CD8−), associated with lymphocytes with a greater degree of atypia, pleomorphism, and larger size; these lymphocytes adopted an angiocentric distribution and acquired a cytotoxic profile with TIA-1 expression. Looking at the clinical course both of our case and of the previous cases, we can state that this phenomenon may be associated with disease progression, although more cases are required to be able to draw significant conclusions.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Vargas Nevado Á, López Navarro N, Gallego Domínguez E, Herrera Ceballos E. Cambio de inmunofenotipo asociado a angiocentricidad y características citotóxicas en un caso de micosis fungoide. Actas Dermosifiliogr. 2016;107:697–699.