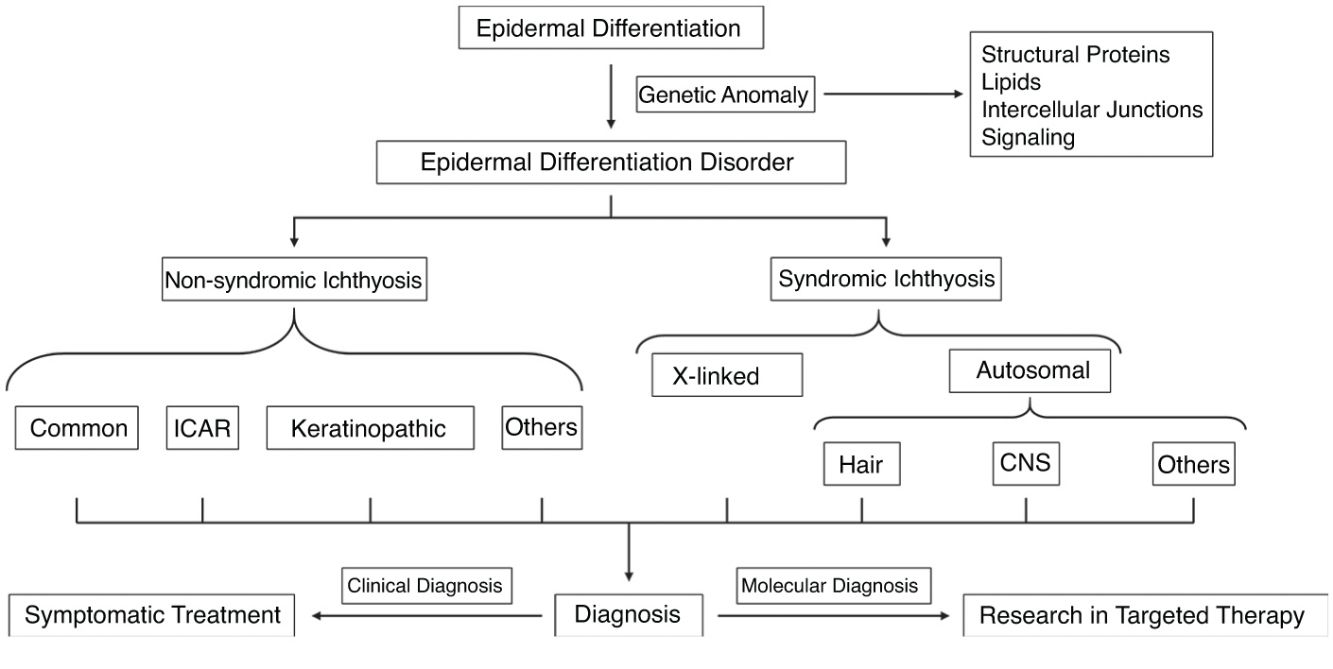
Syndromic ichthyoses are a group of disorders whose genetic alterations impact both epidermal and non-epidermal tissues. Therefore, patients present symptoms in other organs. Most are extraordinary and, in some, ichthyosiform desquamation has been poorly described. Their patterns of inheritance are diverse; their extracutaneous clinical signs, heterogeneous; and the skin symptoms, highly variable, which hinders a proper clinical classification.
Ichthyosis diagnosis starts with proper anamnesis, detailed physical examination, and detection of associated analytic and/or histologic findings. Genetic testing is indispensable, not only for diagnostic certainty, but also because understanding the molecular substrate for each patient is the first step towards finding an individualized therapeutic regimen. While it will almost invariably involve facilitating desquamation and maintaining skin hydration using topical exfoliants and emollients, recently, replacement therapies aiming at substituting the proteins and lipids specifically altered in each patient are being developed and gene therapy approaches with the ultimate goal of curing the disease are being assessed. In part 2 of this review, we will be updating the clinical and genetic findings of syndromic entities, ichthyosis diagnosis and treatment.
Las ictiosis sindrómicas son un grupo de enfermedades cuyas alteraciones genéticas tienen repercusión tanto en los tejidos epidérmicos como en los no epidérmicos, por lo que los pacientes presentan clínica cutánea y síntomas en varios órganos o aparatos. La mayoría son excepcionales, y en algunas de ellas la descamación ictiosiforme ha sido pobremente caracterizada. Su patrón hereditario es diverso, sus manifestaciones clínicas extracutáneas heterogéneas y la afectación cutánea muy variable, lo cual dificulta una buena subclasificación clínica.
El diagnóstico de las ictiosis reside en una buena anamnesis, una exploración física detallada, y la detección de hallazgos analíticos y/o histológicos asociados. El estudio genético es imprescindible, no solo para alcanzar el diagnóstico de certeza, sino porque conocer el sustrato molecular concreto en cada paciente es el primer paso para encontrar un régimen terapéutico individualizado. El tratamiento pasa por eliminar las escamas y mantener la piel hidratada mediante el uso de terapia tópica con exfoliantes y emolientes. Además, en la actualidad, se están desarrollando terapias de reemplazo dirigidas a sustituir las proteínas y los lípidos específicamente alterados en cada entidad, y se están empezando a evaluar estrategias de terapia génica cuya finalidad última es la curación. En la segunda parte de este trabajo hacemos una actualización clínica y genética de las entidades sindrómicas, así como una actualización del diagnóstico y del tratamiento de las ictiosis.
Syndromic ichthyoses are a group of diseases whose genetic alterations affect both epidermal and non-epidermal tissues, which is why patients present with symptoms in other organs and systems. Most are exceptional and, in some of them, the ichthyosiform desquamation has been poorly characterized, so we will only detail the most relevant forms (Table 1). They are subdivided based on the inheritance pattern and the most relevant extracutaneous signs.1
Classification of syndromic ichthyoses.
| Syndromic ichthyoses |
| X-linked syndromic ichthyoses (ORPHA: 281210) |
| X-linked recessive syndromic ichthyosis (ORPHA: 281090) |
| IFAP syndrome (ORPHA: 2273) |
| Chondrodysplasia punctata type 2 (CDPX2, ORPHA: 35173) |
| MEND syndrome (ORPHA: 401973) |
| CHILD syndrome (ORPHA: 139) |
| Autosomal syndromic ichthyoses with prominent hair anomalies (ORPHA: 281222) |
| Netherton syndrome (ORPHA: 634) |
| SAM syndrome (ORPHA: 369992) |
| IHS syndrome (ORPHA: 91132) |
| ILVASC syndrome (ORPHA: 59303) |
| Trichothiodystrophy (ORPHA: 33364) |
| Autosomal syndromic ichthyoses with prominent neurological signs (ORPHA: 281238 and ORPHA: 281241) |
| Sjögren–Larsson syndrome (ORPHA: 816) |
| Refsum disease (ORPHA: 773) |
| CEDNIK syndrome (ORPHA: 66631) |
| MEDNIK syndrome (ORPHA: 171851) |
| Ichthyotic keratoderma–spastic paraplegia–hypomyelination–dysmorphic facies syndrome (ORPHA: –) |
| Congenital ichthyosis–intellectual disability–spastic quadriplegia syndrome (ORPHA: 352333) |
| ARC or ARKID syndrome (ORPHA: 2697) |
| Fetal Gaucher disease (ORPHA: 85212) |
| Multiple sulfatase deficiency (ORPHA: 585) |
| Neu–Laxova syndrome (ORPHA: 2671) |
| Harlequin-like ichthyosis with thrombocytopenia (ORPHA: –) |
| Congenital disorders of glycosylation (ORPHA: 137) |
| Other syndromic ichthyoses (ORPHA: 281244) |
| KID syndrome (ORPHA: 477) |
| Neutral lipid storage disease (ORPHA: 98907) |
| Ichthyosis-prematurity syndrome (ORPHA: 88621) |
| HELIX syndrome (ORPHA: 528105) |
| Ichthyosis–short stature–brachydactyly–microspherophakia syndrome (ORPHA: 363992) |
| Palmoplantar and perianal keratoderma/ichthyosis-like |
IFAP: ichthyosis follicularis, alopecia, and photophobia; MEND: male EBP disorder with neurological defects; CHILD: congenital hemidysplasia with ichthyosiform erythroderma and limb defects; SAM: severe dermatitis, multiple allergies, and metabolic wasting; ILVASC: ichthyosis, leukocyte vacuoles, alopecia, and sclerosing cholangitis; CEDNIK: cerebral dysgenesis, neuropathy, ichthyosis, and keratoderma; MEDNIK: intellectual disability, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma; ARC: arthrogryposis, renal dysfunction, and cholestasis; ARKID: autosomal recessive keratoderma, ichthyosis, and deafness; KID: keratitis, ichthyosis, and deafness; HELIX: hypohidrosis, electrolyte imbalance, lacrimal gland dysfunction, ichthyosis, and xerostomia.
All causal genes in this group affect cholesterol synthesis.
- -
Syndromic X-linked ichthyosis: patients present with a cutaneous phenotype similar to the non-syndromic form, but with variable associated extracutaneous symptoms that may include anosmia, hypogonadotropic hypogonadism – Kallman syndrome – and intellectual disability. It is due to large hemizygous microdeletions affecting both STS2 and contiguous genes, whose absence determines the associated clinical spectrum. Strictly, this type of ichthyosis could be considered non-syndromic since the extracutaneous symptoms are not due to STS deficiency, but by the additional deletion of contiguous genes.
- -
IFAP syndrome (ichthyosis follicularis–alopecia–photophobia): characterized by generalized cutaneous affectation with erythema, follicular hyperkeratosis, palmoplantar keratoderma, alopecia and photophobia. Additionally, some patients exhibit intellectual disability, corneal opacity, renal dysplasia, cryptorchidism and skeletal malformations (BRESHECK syndrome). It is due to recessive pathogenic variants in MBTPS2.3 Of note, a phenotypically similar entity called hereditary mucoepithelial dysplasia – also called IFAP2 – exists and is due to dominant pathogenic variants in SREBF1,4 a gene found not in the X chromosome, but in chromosome 17 (Fig. 1). Both genes are transcription factors that activate the cholesterol synthesis pathway.
- -
Chondrodysplasia punctata type 2: this disease is lethal in men and exhibits a peculiar phenotype of ichthyosis along the Blaschko lines due to X chromosome inactivation. Typical symptoms include cataracts, skeletal abnormalities like low height and rhizomelic limb shortening and punctate appearance in long bone epiphysis radiographs. It is due to dominant pathogenic variants in EBP,5 which encodes a cholesterol synthesis enzyme.
This group is characterized by patients with characteristic hair abnormalities, a clinical finding that can help with disease diagnosis. However, both the clinical signs and molecular and etiopathogenic causes are very diverse.
- -
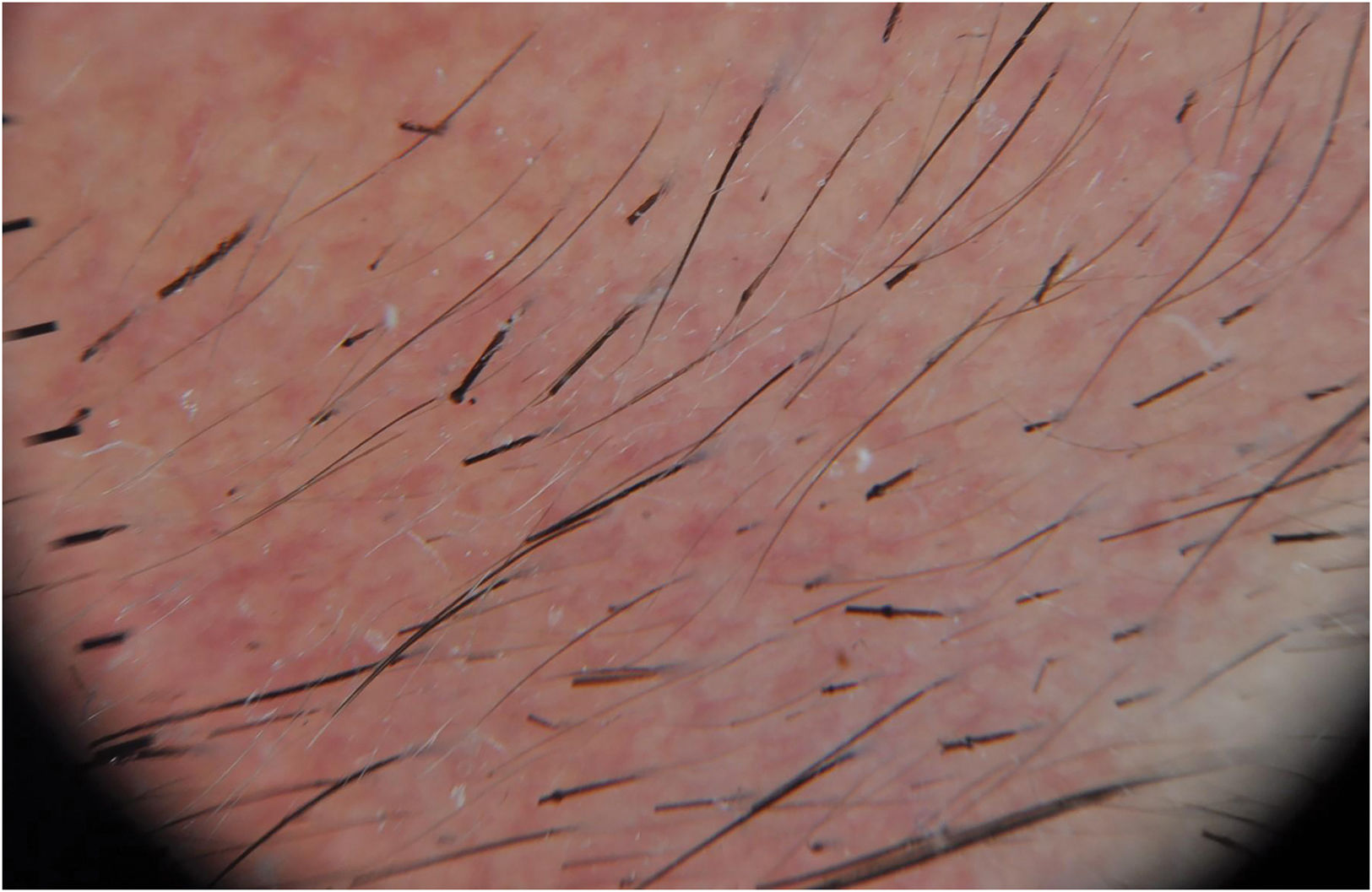
Netherton syndrome: Probably the most common syndromic ichthyosis, and the easiest one to diagnose when hair shaft alterations are identified. Although some cases describe neonatal presentation as a collodion baby, most children are born with erythroderma and a severe alteration of the cutaneous barrier causing recurrent episodes of hypernatremic dehydration and sepsis. Facial erythema is characteristic in these patients, who often exhibit intense and incoercible pruritus. In time, patients may develop a characteristic cutaneous presentation known as ichthyosis linearis circumflexa, characterized by arch-shaped or polylobulated lesions with double-edged scaling (Fig. 2). Most patients present with trichorrhexis invaginata also known as bamboo hair, a hair shaft disorder that may affect head hair as well as eyebrows and eyelashes and can be found during exploratory dermoscopy (Fig. 3). Consequently, patient hair is short and uneven, since it is fragile and breaks easily along the areas with trichorrhexis invaginata. Many patients also suffer from atopic diathesis, with skin eczematiform flares and food allergies, as well as verruciform lesions and a predisposition to human papillomavirus cutaneous infections. Netherton syndrome is caused by recessive pathogenic variants in SPINK5.6 It can be diagnosed by skin biopsy immunohistochemistry, since patients show a lack of LEKTI expression (the protein encoded by this gene, localized in the stratum corneum). Its absence prevents kallikrein inactivation, epidermal enzymes responsible for corneocyte degradation needed for physiological desquamation.
- -
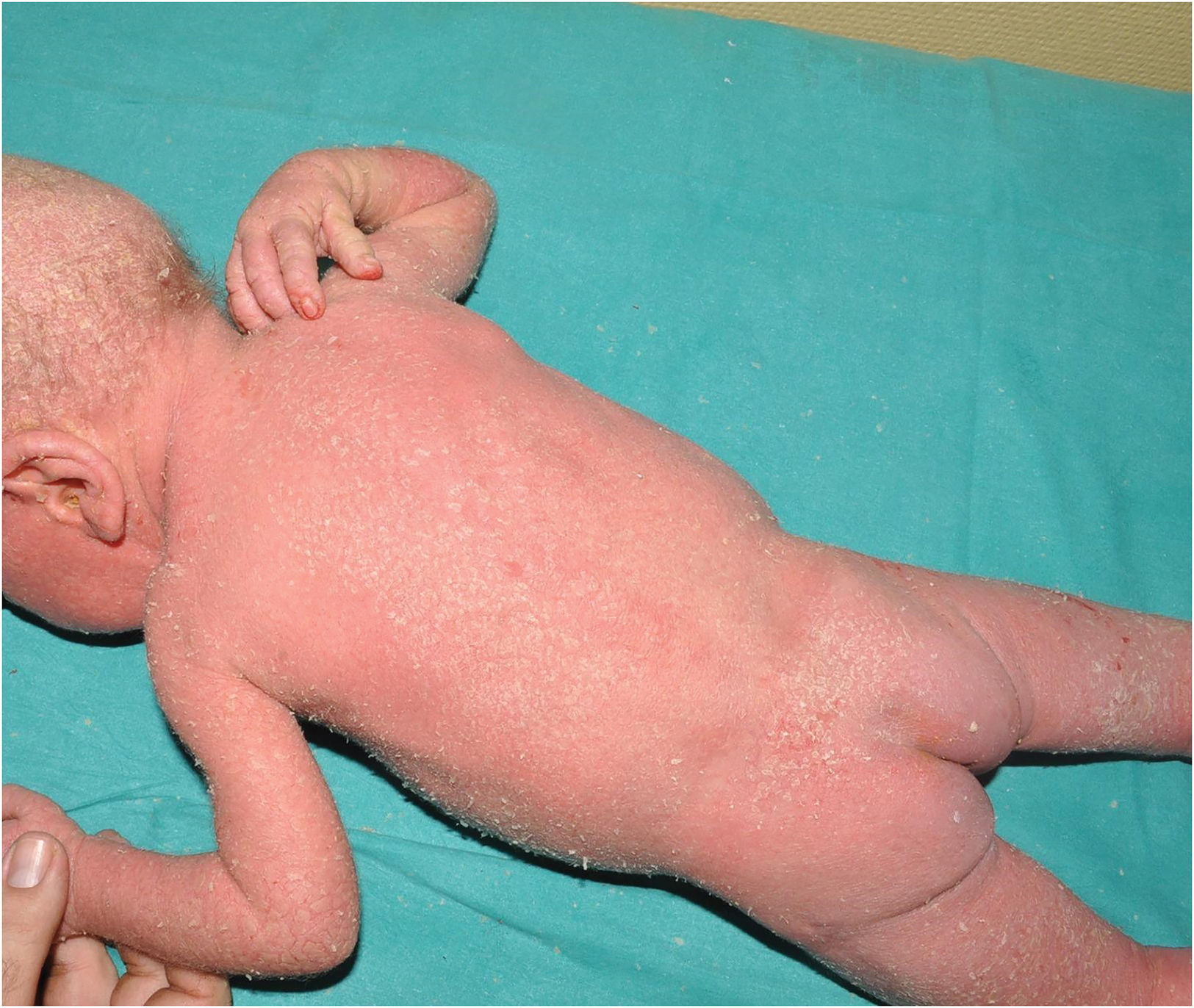
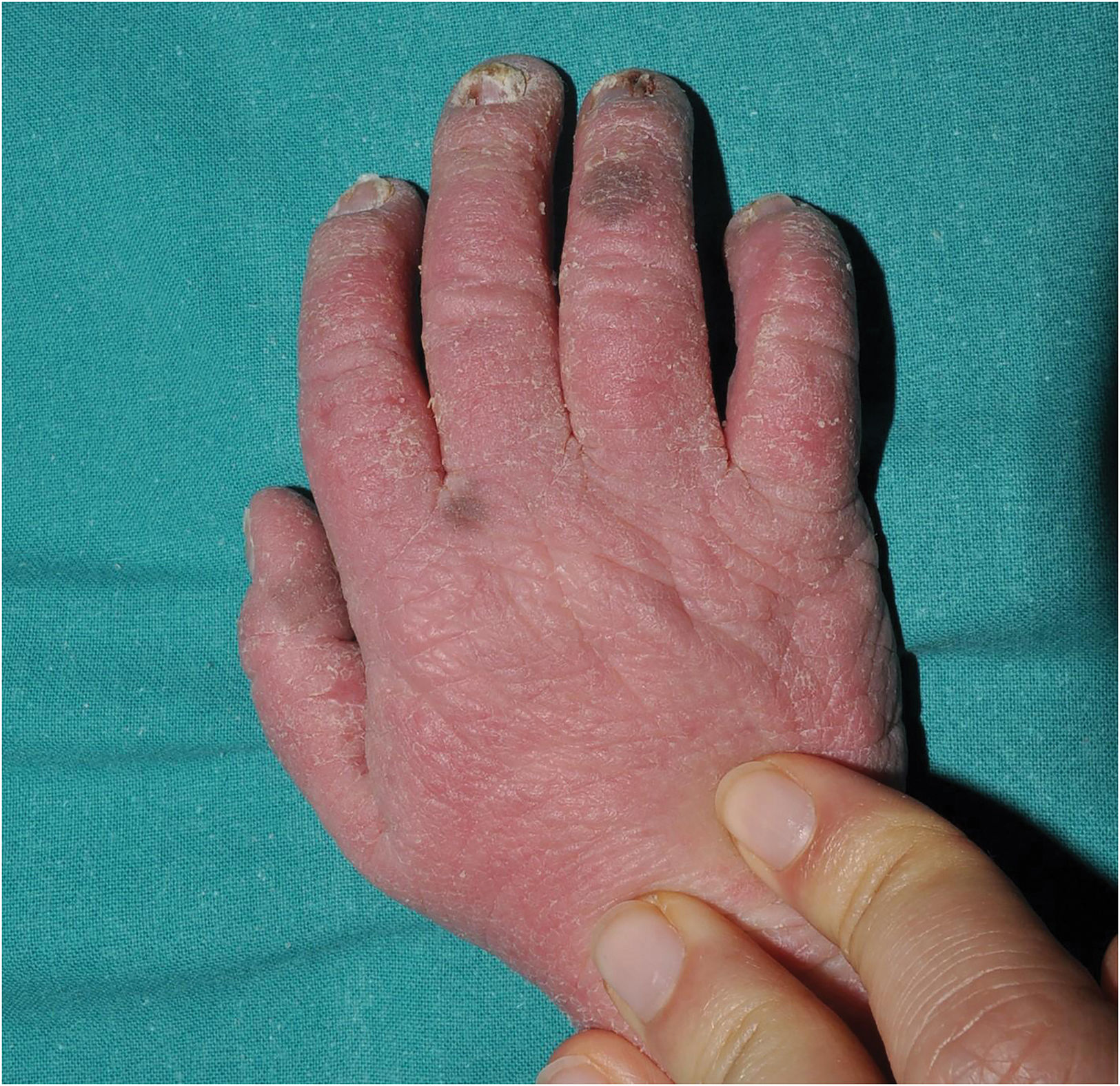
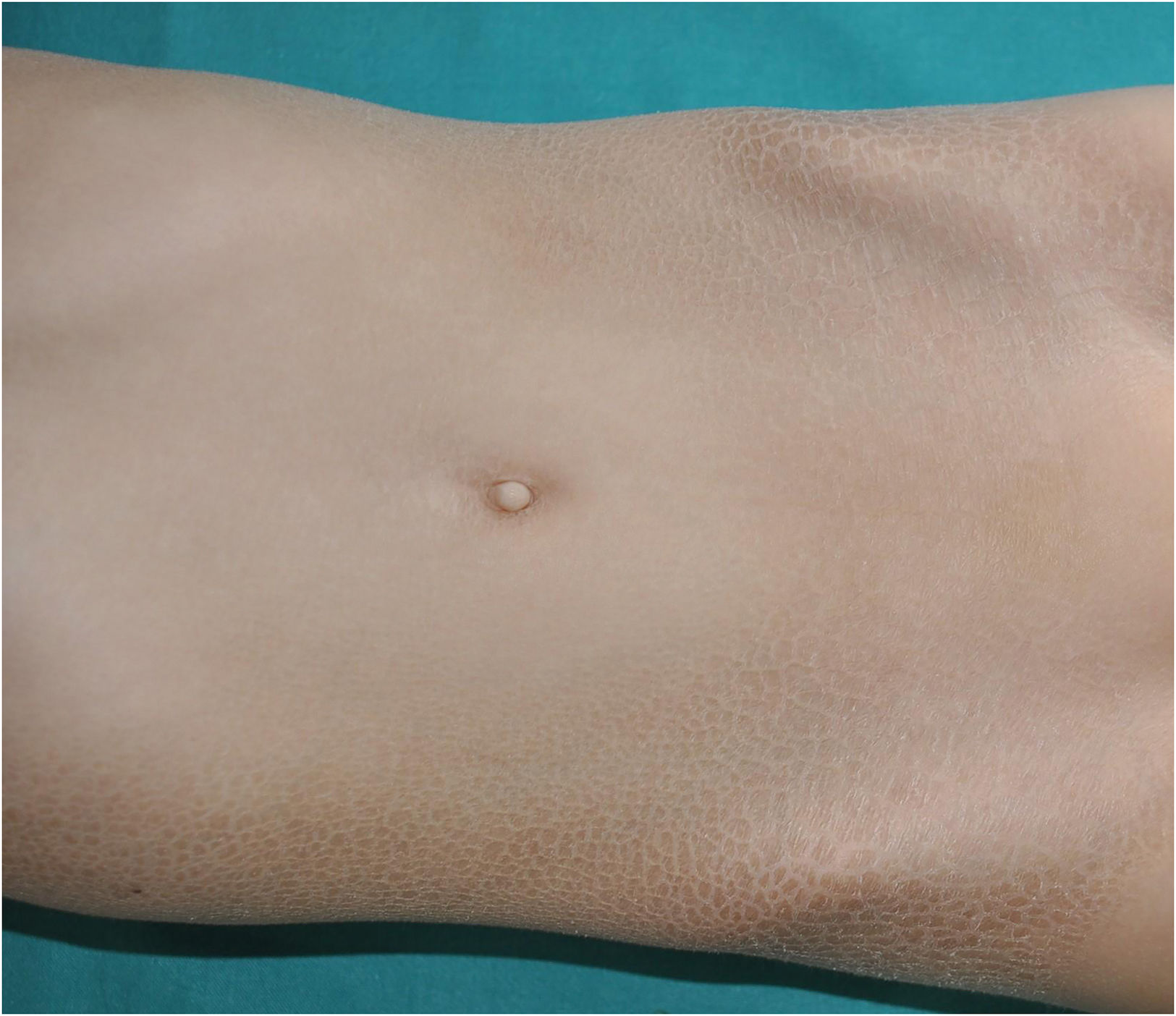
SAM syndrome (severe dermatitis–multiple allergies–metabolic wasting syndrome): A desmosomal disease whose acronym summarizes its essential clinical characteristics: severe dermatitis with ichthyosiform erythroderma, allergies and metabolic wasting leading to failure to thrive. Since SAM syndrome was described after the last consensus classification, it is not a part of it. However, we believe that its physiopathological origin and clinical signs justify the inclusion of SAM syndrome in the group of syndromic ichthyoses with hair abnormalities. Patients present symptoms since birth, often with repeated life-threatening systemic infections. Cutaneous symptoms include severe desquamating erythroderma with hypotrichosis and onychodystrophy (Fig. 4). Tooth enamel anomalies and transient hyperpigmented lesions whose histology suggest a post-inflammatory origin are not rare findings (Fig. 5). SAM syndrome is due to recessive pathogenic variants in DSG17 or dominant pathogenic variants in DSP.8 This last case may associate cardiomyopathy, since DSP is expressed in heart muscle.9
- -
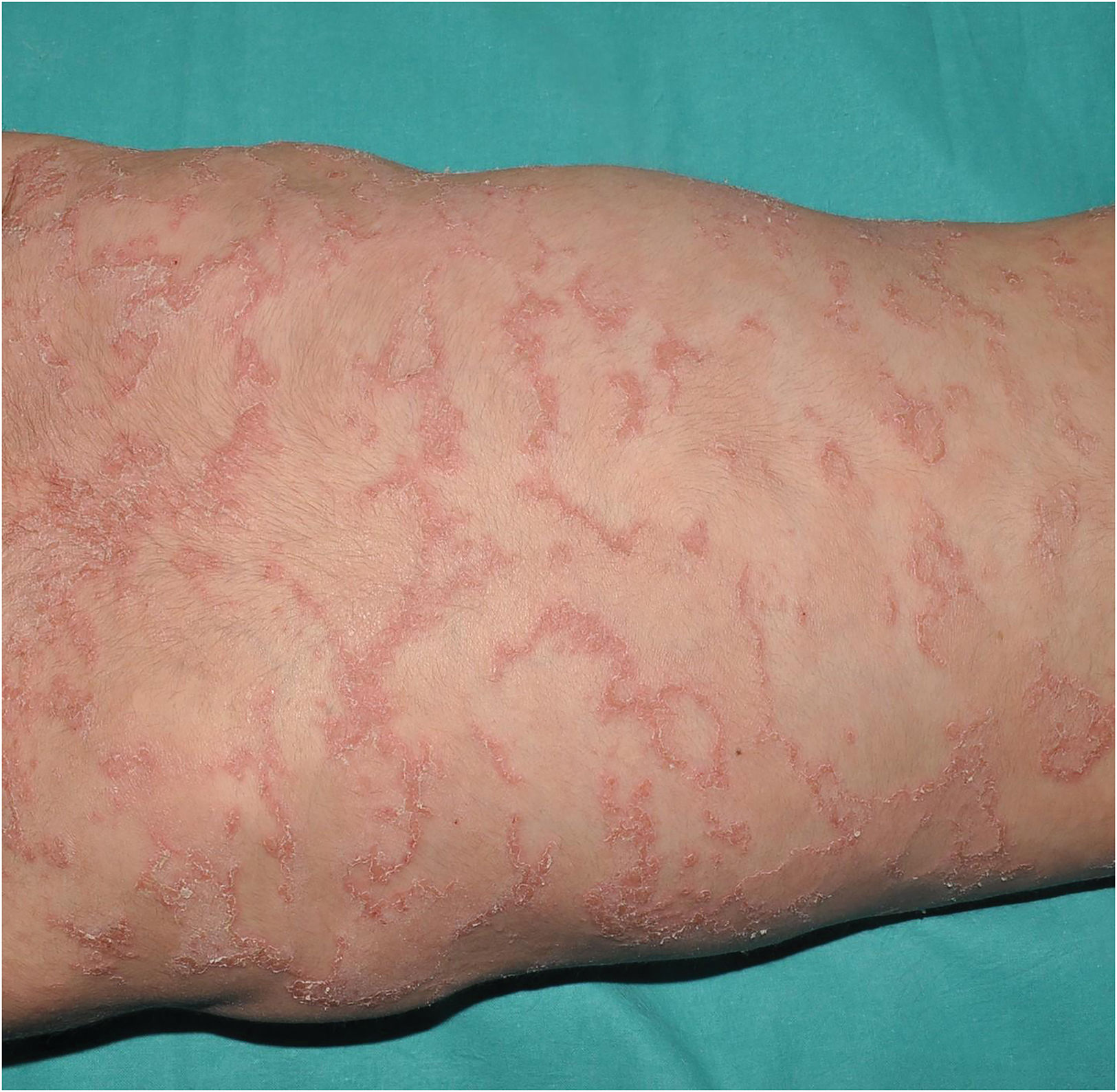
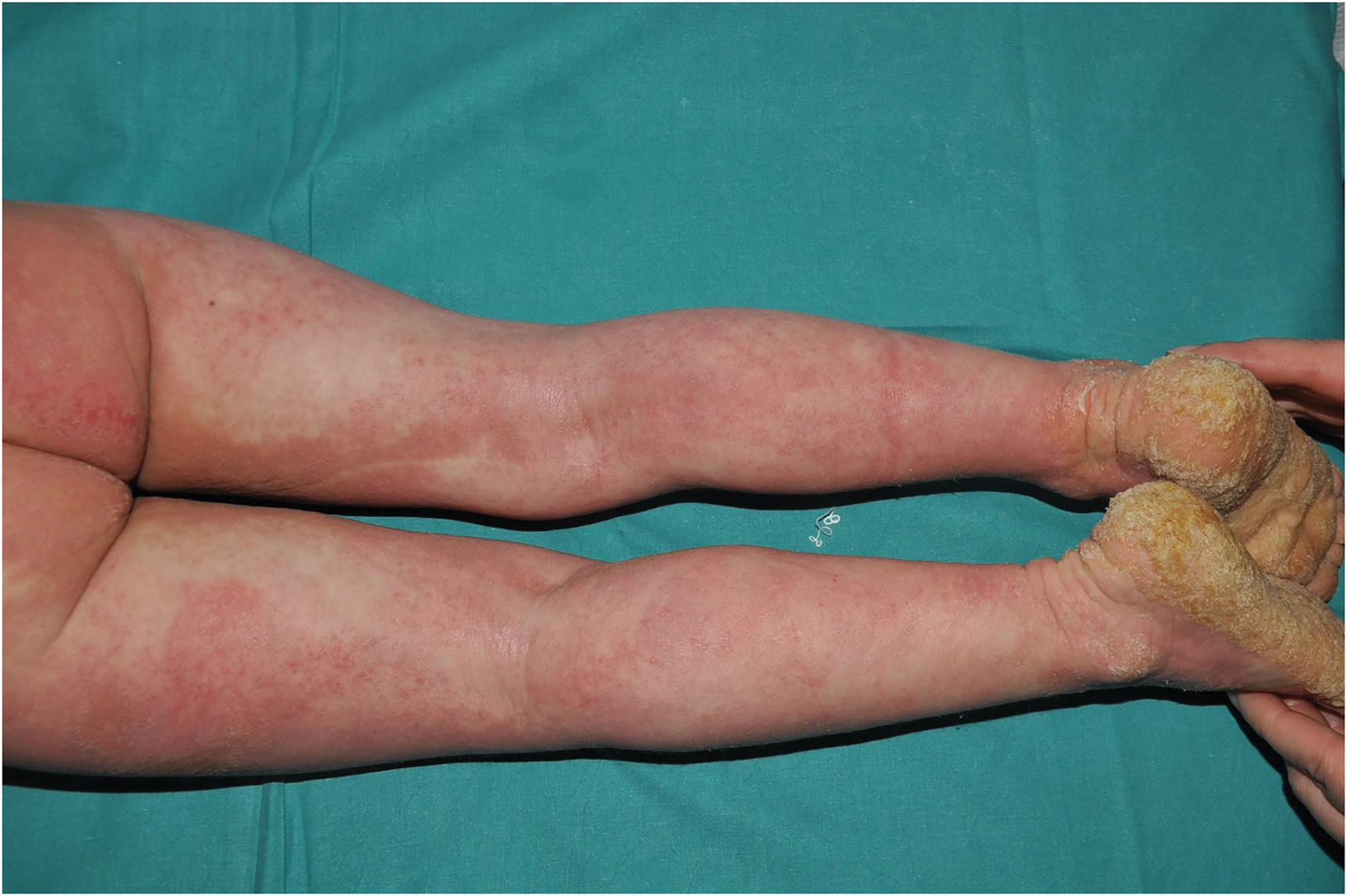
Trichothiodystrophy: characterized by ichthyosis coupled with fragile hair and nails with sulfur deficiency. Ichthyosiform desquamation is usually mild, dark and relatively large, with forehead and trunk affectation (Fig. 6). Characteristically, alternating light and dark bands can be observed in the hair shaft under polarized light (tiger-tail banding). It is due to recessive pathogenic variants in AARS1,10GTF2E2,11MAARS1,10MPLKIP,12RNF113A,13 or TARS1,14 which code proteins involved in protein translation. There is a subtype which also presents photosensitivity, progressive neuropathy and accelerated ageing, caused by recessive pathogenic variants in ERCC2,15ERCC3,16 or GTF2H5,17 which code proteins involved in DNA repair as well as protein translation. Same as the previous cases, this group shows a striking molecular heterogeneity that hinders geno-phenotypic correlation.
The most common disease in this group is Sjögren–Larsson syndrome, characterized by intellectual disability, spasticity and eye abnormalities (crystalline retinopathy), which is highly suggestive for diagnosis. Cutaneous involvement is not very significant and consists of discrete generalized hyperkeratosis which, in some areas, determines parallel linear lesions known as railway track lesions which can be reminiscent of lichenification in the flexures. Many patients show severe pruritus. It is due to recessive pathogenic variants in ALDH3A2,18 a fatty aldehyde dehydrogenase responsible for synthesis of fatty acids used in ceramide synthesis.
Other syndromic ichthyosesThis group includes entities that do not respond to the characteristics of the previous groups.
- -
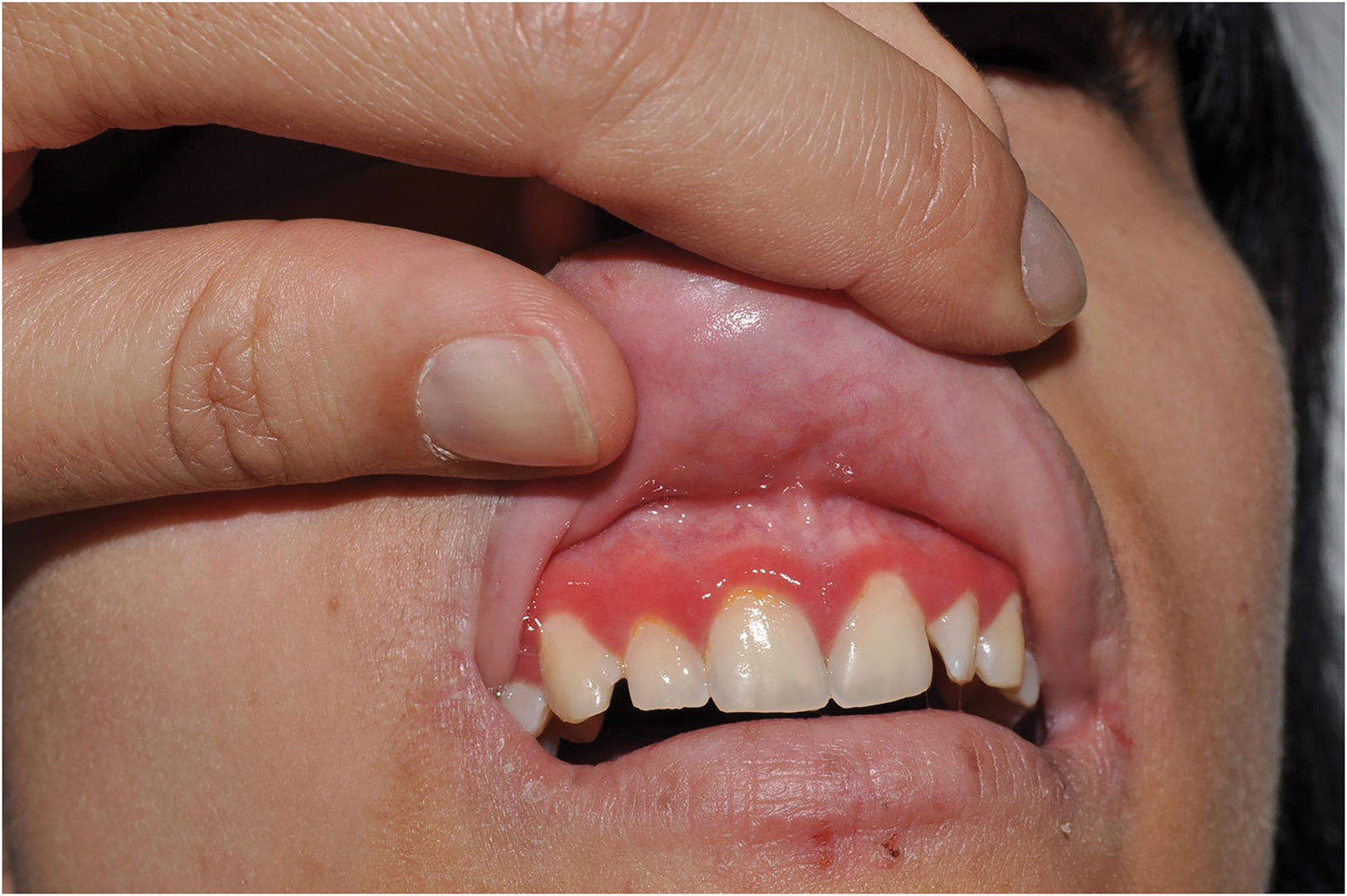
KID syndrome (keratitis–ichthyosis–deafness): Characterized by keratitis, hyperkeratosis espinulosa, palmoplantar keratoderma and sensorineural deafness. It is caused by dominant pathogenic variants in GJB219 or GJB6,20 or recessive pathogenic variants in AP1B1,21 which encode gap junction proteins in charge of intercellular communication and the clathrin-coated vesicle adaptor complex, respectively. Additionally, postzygotic GJB2 variants cause keratotic lesions along the Blaschko lines that may be transmitted to the offspring as KID syndrome if it affects the gonads.22 These mosaic forms have less systemic impact since they spare sight and hearing (Fig. 7).
- -
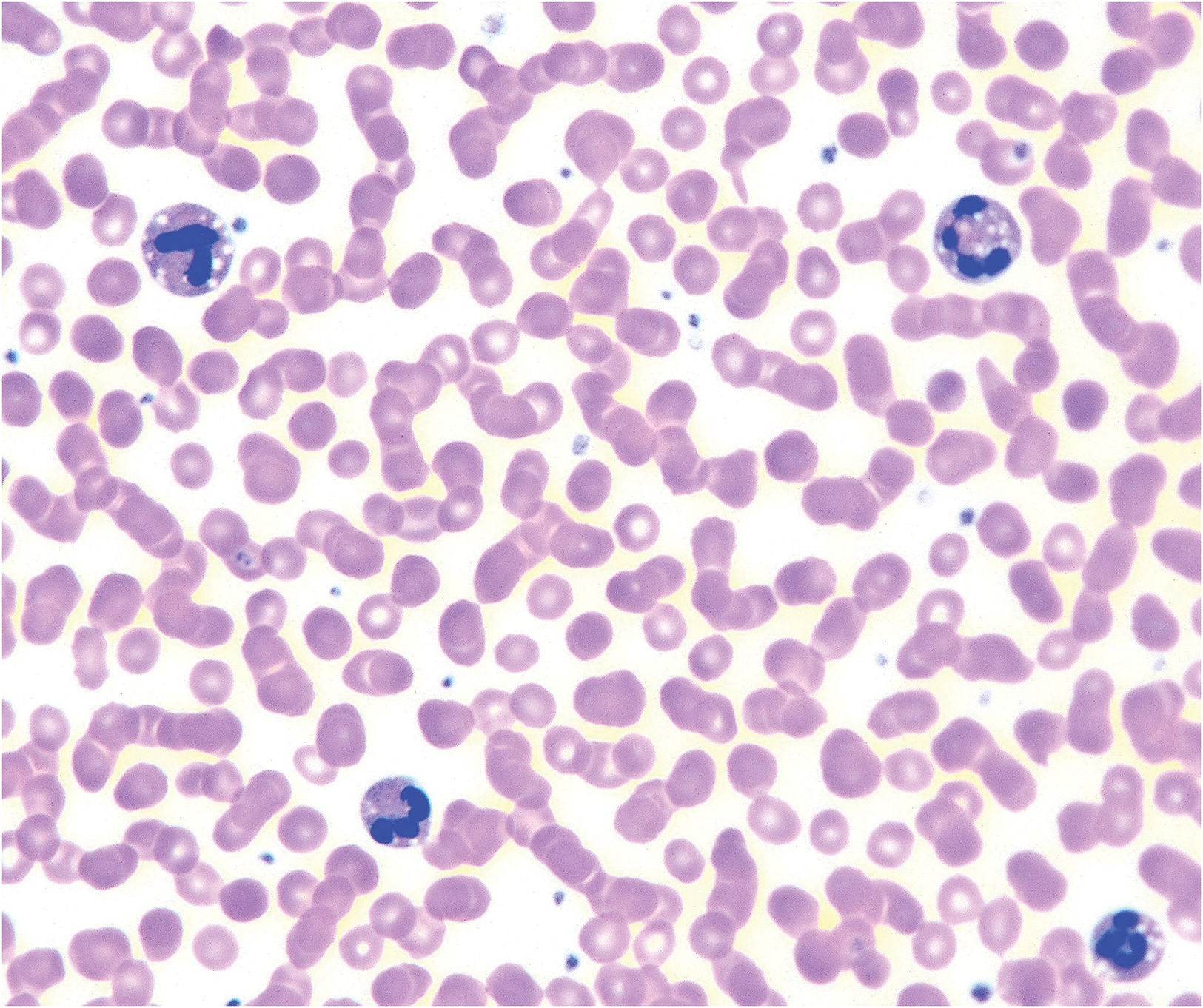
Neutral lipid storage disease: Also known as Chanarin–Dorfman disease, it presents with mild congenital ichthyosiform erythroderma, hepatosplenomegaly, myopathy, hypoacusia and cataracts.23 Histologically, vacuole accumulation is seen in most tissues (Fig. 8). It is due to recessive pathogenic variants in ABHD5,24 encoding an enzyme that synthesizes linoleic acid used in ceramide synthesis and whose absence leads to intracellular neutral lipid storage.
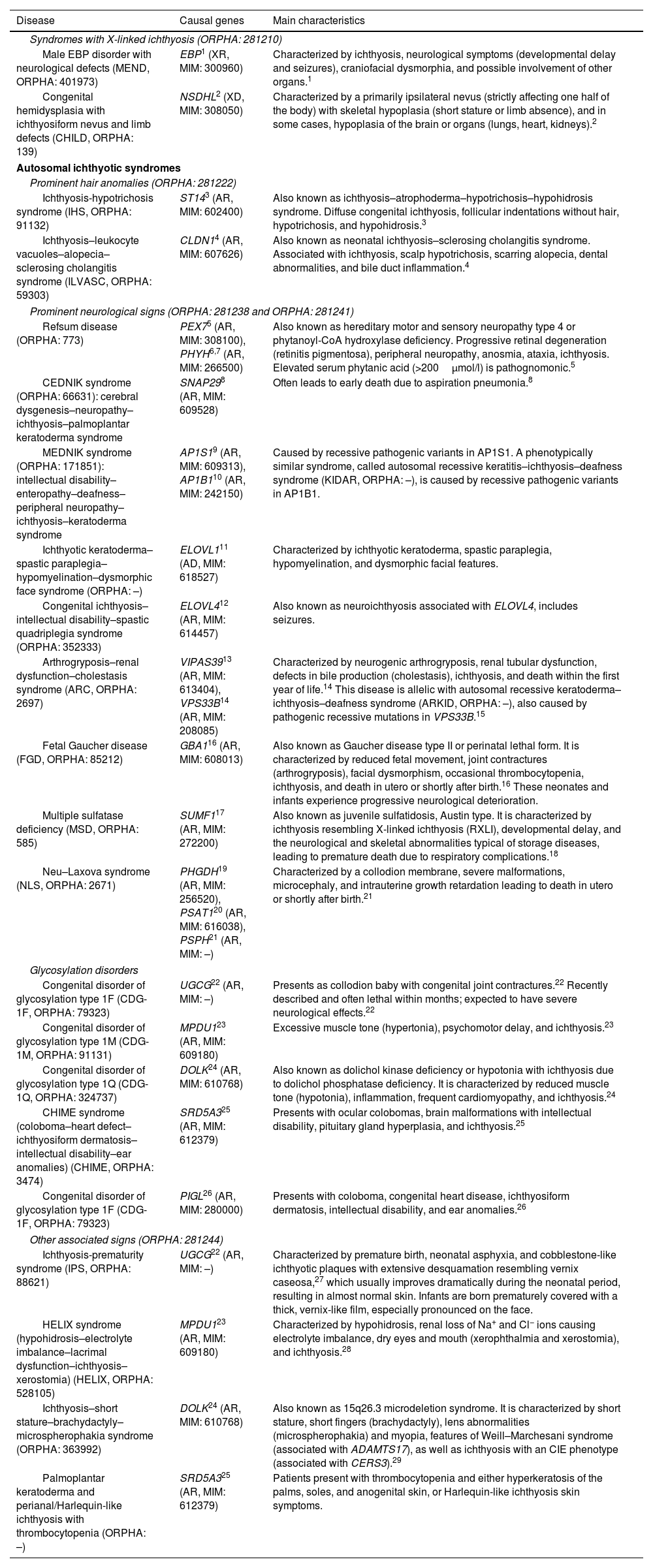
The main characteristics of the most infrequent syndromic ichthyoses are shown in Table 2.
Rare forms of syndromic ichthyoses.
| Disease | Causal genes | Main characteristics |
|---|---|---|
| Syndromes with X-linked ichthyosis (ORPHA: 281210) | ||
| Male EBP disorder with neurological defects (MEND, ORPHA: 401973) | EBP1 (XR, MIM: 300960) | Characterized by ichthyosis, neurological symptoms (developmental delay and seizures), craniofacial dysmorphia, and possible involvement of other organs.1 |
| Congenital hemidysplasia with ichthyosiform nevus and limb defects (CHILD, ORPHA: 139) | NSDHL2 (XD, MIM: 308050) | Characterized by a primarily ipsilateral nevus (strictly affecting one half of the body) with skeletal hypoplasia (short stature or limb absence), and in some cases, hypoplasia of the brain or organs (lungs, heart, kidneys).2 |
| Autosomal ichthyotic syndromes | ||
| Prominent hair anomalies (ORPHA: 281222) | ||
| Ichthyosis-hypotrichosis syndrome (IHS, ORPHA: 91132) | ST143 (AR, MIM: 602400) | Also known as ichthyosis–atrophoderma–hypotrichosis–hypohidrosis syndrome. Diffuse congenital ichthyosis, follicular indentations without hair, hypotrichosis, and hypohidrosis.3 |
| Ichthyosis–leukocyte vacuoles–alopecia–sclerosing cholangitis syndrome (ILVASC, ORPHA: 59303) | CLDN14 (AR, MIM: 607626) | Also known as neonatal ichthyosis–sclerosing cholangitis syndrome. Associated with ichthyosis, scalp hypotrichosis, scarring alopecia, dental abnormalities, and bile duct inflammation.4 |
| Prominent neurological signs (ORPHA: 281238 and ORPHA: 281241) | ||
| Refsum disease (ORPHA: 773) | PEX75 (AR, MIM: 308100), PHYH6,7 (AR, MIM: 266500) | Also known as hereditary motor and sensory neuropathy type 4 or phytanoyl-CoA hydroxylase deficiency. Progressive retinal degeneration (retinitis pigmentosa), peripheral neuropathy, anosmia, ataxia, ichthyosis. Elevated serum phytanic acid (>200μmol/l) is pathognomonic.5 |
| CEDNIK syndrome (ORPHA: 66631): cerebral dysgenesis–neuropathy–ichthyosis–palmoplantar keratoderma syndrome | SNAP298 (AR, MIM: 609528) | Often leads to early death due to aspiration pneumonia.8 |
| MEDNIK syndrome (ORPHA: 171851): intellectual disability–enteropathy–deafness–peripheral neuropathy–ichthyosis–keratoderma syndrome | AP1S19 (AR, MIM: 609313), AP1B110 (AR, MIM: 242150) | Caused by recessive pathogenic variants in AP1S1. A phenotypically similar syndrome, called autosomal recessive keratitis–ichthyosis–deafness syndrome (KIDAR, ORPHA: –), is caused by recessive pathogenic variants in AP1B1. |
| Ichthyotic keratoderma–spastic paraplegia–hypomyelination–dysmorphic face syndrome (ORPHA: –) | ELOVL111 (AD, MIM: 618527) | Characterized by ichthyotic keratoderma, spastic paraplegia, hypomyelination, and dysmorphic facial features. |
| Congenital ichthyosis–intellectual disability–spastic quadriplegia syndrome (ORPHA: 352333) | ELOVL412 (AR, MIM: 614457) | Also known as neuroichthyosis associated with ELOVL4, includes seizures. |
| Arthrogryposis–renal dysfunction–cholestasis syndrome (ARC, ORPHA: 2697) | VIPAS3913 (AR, MIM: 613404), VPS33B14 (AR, MIM: 208085) | Characterized by neurogenic arthrogryposis, renal tubular dysfunction, defects in bile production (cholestasis), ichthyosis, and death within the first year of life.14 This disease is allelic with autosomal recessive keratoderma–ichthyosis–deafness syndrome (ARKID, ORPHA: –), also caused by pathogenic recessive mutations in VPS33B.15 |
| Fetal Gaucher disease (FGD, ORPHA: 85212) | GBA116 (AR, MIM: 608013) | Also known as Gaucher disease type II or perinatal lethal form. It is characterized by reduced fetal movement, joint contractures (arthrogryposis), facial dysmorphism, occasional thrombocytopenia, ichthyosis, and death in utero or shortly after birth.16 These neonates and infants experience progressive neurological deterioration. |
| Multiple sulfatase deficiency (MSD, ORPHA: 585) | SUMF117 (AR, MIM: 272200) | Also known as juvenile sulfatidosis, Austin type. It is characterized by ichthyosis resembling X-linked ichthyosis (RXLI), developmental delay, and the neurological and skeletal abnormalities typical of storage diseases, leading to premature death due to respiratory complications.18 |
| Neu–Laxova syndrome (NLS, ORPHA: 2671) | PHGDH19 (AR, MIM: 256520), PSAT120 (AR, MIM: 616038), PSPH21 (AR, MIM: –) | Characterized by a collodion membrane, severe malformations, microcephaly, and intrauterine growth retardation leading to death in utero or shortly after birth.21 |
| Glycosylation disorders | ||
| Congenital disorder of glycosylation type 1F (CDG-1F, ORPHA: 79323) | UGCG22 (AR, MIM: –) | Presents as collodion baby with congenital joint contractures.22 Recently described and often lethal within months; expected to have severe neurological effects.22 |
| Congenital disorder of glycosylation type 1M (CDG-1M, ORPHA: 91131) | MPDU123 (AR, MIM: 609180) | Excessive muscle tone (hypertonia), psychomotor delay, and ichthyosis.23 |
| Congenital disorder of glycosylation type 1Q (CDG-1Q, ORPHA: 324737) | DOLK24 (AR, MIM: 610768) | Also known as dolichol kinase deficiency or hypotonia with ichthyosis due to dolichol phosphatase deficiency. It is characterized by reduced muscle tone (hypotonia), inflammation, frequent cardiomyopathy, and ichthyosis.24 |
| CHIME syndrome (coloboma–heart defect–ichthyosiform dermatosis–intellectual disability–ear anomalies) (CHIME, ORPHA: 3474) | SRD5A325 (AR, MIM: 612379) | Presents with ocular colobomas, brain malformations with intellectual disability, pituitary gland hyperplasia, and ichthyosis.25 |
| Congenital disorder of glycosylation type 1F (CDG-1F, ORPHA: 79323) | PIGL26 (AR, MIM: 280000) | Presents with coloboma, congenital heart disease, ichthyosiform dermatosis, intellectual disability, and ear anomalies.26 |
| Other associated signs (ORPHA: 281244) | ||
| Ichthyosis-prematurity syndrome (IPS, ORPHA: 88621) | UGCG22 (AR, MIM: –) | Characterized by premature birth, neonatal asphyxia, and cobblestone-like ichthyotic plaques with extensive desquamation resembling vernix caseosa,27 which usually improves dramatically during the neonatal period, resulting in almost normal skin. Infants are born prematurely covered with a thick, vernix-like film, especially pronounced on the face. |
| HELIX syndrome (hypohidrosis–electrolyte imbalance–lacrimal dysfunction–ichthyosis–xerostomia) (HELIX, ORPHA: 528105) | MPDU123 (AR, MIM: 609180) | Characterized by hypohidrosis, renal loss of Na+ and Cl− ions causing electrolyte imbalance, dry eyes and mouth (xerophthalmia and xerostomia), and ichthyosis.28 |
| Ichthyosis–short stature–brachydactyly–microspherophakia syndrome (ORPHA: 363992) | DOLK24 (AR, MIM: 610768) | Also known as 15q26.3 microdeletion syndrome. It is characterized by short stature, short fingers (brachydactyly), lens abnormalities (microspherophakia) and myopia, features of Weill–Marchesani syndrome (associated with ADAMTS17), as well as ichthyosis with an CIE phenotype (associated with CERS3).29 |
| Palmoplantar keratoderma and perianal/Harlequin-like ichthyosis with thrombocytopenia (ORPHA: –) | SRD5A325 (AR, MIM: 612379) | Patients present with thrombocytopenia and either hyperkeratosis of the palms, soles, and anogenital skin, or Harlequin-like ichthyosis skin symptoms. |
AD: autosomal dominant; AR: autosomal recessive; HD: homozygous deletion; XD: X-linked dominant; XR: X-linked recessive.
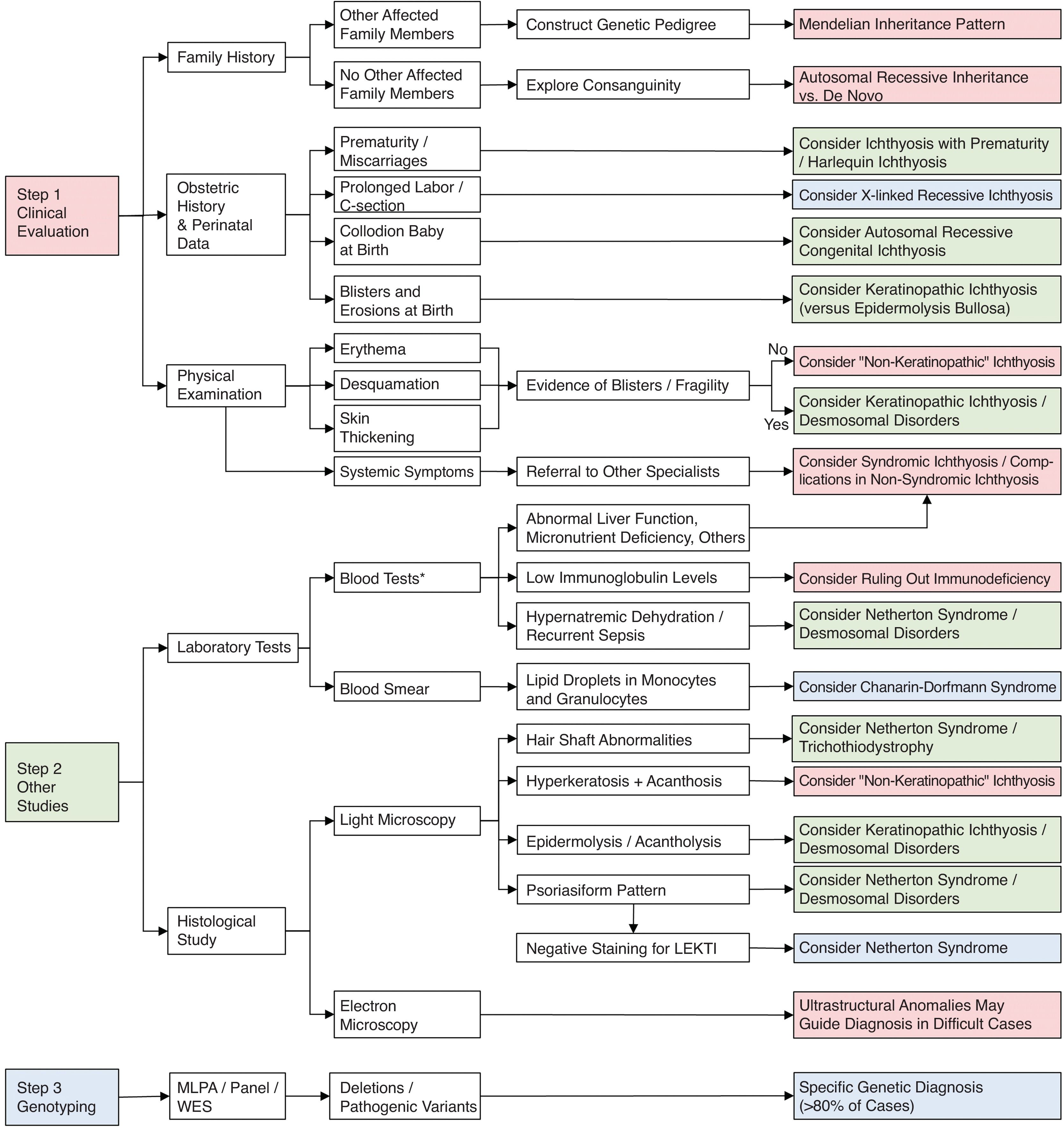
Generalized alteration of the cutaneous barrier in patients with disorders of epidermal differentiation disorders (EDD) is present since birth or within the first few months of life, which helps clinical diagnosis. However, the poor phenotype–genotype correlation hinders a more precise diagnosis in many cases. Fig. 9 shows a diagnostic algorithm in which adequate anamnesis is of paramount importance.
Blood tests, including red blood cells, hepatic and renal function, blood electrolyte levels, immunoglobulin serum levels and blood smear can help exclude syndromic forms of ichthyosis and their associated anomalies.25 For example, Netherton syndrome and desmosomal disorders both carry a risk of hypernatremic dehydration in babies.7,26 Moreover, serum immunoglobulin levels can be useful to establish differential diagnosis with hereditary immunodeficiencies, which can also present erythema and desquamation. Referring the patient to other specialists based on the clinical findings should be considered.25 Chanarin–Dorfman presents lipidic droplets in granulocytes and monocytes in blood smear (known as Jordan's anomaly)27 (Fig. 8).
Biopsies for routine histology, immunohistochemistry or, more rarely, electron microscopy may help with differential diagnosis.28 Absence of LEKTI expression (encoded by SPINK5) in immunohistochemistry can confirm a Netherton syndrome diagnosis29 and is especially useful if genetic analysis is not available.28 Light microscope analysis of the hair shaft is an accessible and non-invasive technique that adds useful information in the ichthyoses associated with hair abnormalities, such as trichorrhexis invaginate in Netherton syndrome or “tiger-tail banding” under polarized light in trichothiodystrophy.30 Although clinical diagnosis of ichthyosis is usually easy, phenotype–genotype correlation is often hard to establish. Although genetic testing using next generation sequencing is widely used in developed countries to confirm diagnosis, genetic abnormalities are not found in 15–20% of patients with EDD phenotype.31–35 This can be due to undetectable or undescribed pathogenic variants, the latter being the ones that allow a broadening of the group of ichthyosis when associated with distinctive clinical findings.
Genetic counselingEDD follow patterns of monogenic Mendelian inheritance, which allows for genetic counseling, where information is provided to affected families (patients and relatives) about the molecular mechanisms and odds of transmission to potential offspring. However, perception of the risk of having an affected child changes substantially depending on the disease and a pregnancy may not be considered high-risk by couples if they already have a child with mild symptoms. In contrast, patients with severe forms or a heavy impact on quality of life may ask for genetic counseling to avoid future high-risk pregnancies.36 Regional, cultural, and socioeconomic idiosyncrasies may also influence access to genetic counseling.
Prenatal diagnosis requires obtaining embryonic tissue. Low levels of unconjugated estriol and copy number alterations in maternal serum – which detects deletions in maternal sexual chromosomes – are closely associated win an increased risk of recessive X-linked ichthyosis in male fetuses and can be used, along with other molecular techniques, for prenatal diagnosis.37,38 In any case, the method of choice for prenatal diagnosis is the genetic analysis of known pathogenic variants in the family. Pre-implantation diagnosis may be used by at-risk couples to select unaffected embryos prior to in vitro fertilization, although many countries do not consider it appropriate for ichthyosis.39
TreatmentEDD are genetic diseases that currently lack curative treatment. Therapy is currently aimed at alleviating symptoms to improve the patient's quality of life. Therefore, this requires treatments that not only have to be effective but also safe and well tolerated. However, the reality is that there are few studies on the long-term effects of therapies and their efficacy varies greatly across patients, even among those suffering from the same type of EDD. Therefore, the chosen treatment and administration regimen depend on expert recommendations in clinical practice guidelines, information sharing with patient associations, accessibility to treatment and the personal experience of each individual patient.26,40,41
Topical therapyThe basis of all EDD therapy is topical treatments, which aim to reduce epidermal thickness and facilitate its desquamation, as well as itching, tightness, and cracking, improving skin appearance. They can act at different levels but the objective is always to normalize the epidermal barrier function.42
Bathing and exfoliationBaths aim to soften the scales to facilitate their removal via mechanical exfoliation. Baths should be prolonged and may be supplemented with salts, oils, or bicarbonate to increase hydration and promote exfoliation. Dilute sodium hypochlorite can help control skin infections and decrease odor that can be caused by microbial overgrowth in patients with pronounced hyperkeratosis. Exfoliation can be performed by hand, sponge, file, pumice, or even scissors for larger scales with partial sloughing. This process usually requires between 30 and 60min a day, which is a factor that significantly impacts patient quality of life, as they must prioritize this over other activities such as playing, studying, working, and having leisure time. In EDD with scalp involvement it is important to remove scales and crusts to avoid scarring alopecia, which is often unavoidable despite this care.
EmolientsEmolients aim to maintain a correct water balance, hydrating the epidermis and sealing the barrier to prevent water loss. Their formulation has an impact on the degree of hydration, lubrication, and occlusion they provide. However, the occlusive effect may interfere with sweating, aggravating the heat intolerance suffered by many patients. Vaseline and paraffin are inexpensive and effective emolients but they are cosmetically uncomfortable, which can make other formulations based on urea, propylene glycol, or dexpanthenol preferable. Overall, it is usually necessary to apply them at least twice a day.40
Topical keratolyticsKeratolytics facilitate proteolytic degradation of cytoskeleton and intercellular junctions, which reduces hyperkeratosis and facilitates desquamation. These drugs include urea, salicylic acid, and lactic acid. Adverse effects are usually mild, including itching, burning, and irritation; however, acids can be absorbed and have systemic side effects.43 This is especially serious in the case of salicylic acid, which can cause salicylism with nausea, vomiting, tachypnea, irritability, coma, and even death. Therefore, it is of paramount importance to limit its application, particularly in children, patients with impaired renal or hepatic function and in cases with a high percentage of affected body surface area.44
RetinoidsRetinoids decrease keratinocyte proliferation and differentiation, controlling the number of cells that can form the stratum corneum and reducing hyperkeratosis and inflammation. They are available only in some countries where they are marketed for acne, so they are used off-label. Tazarotene45 and adapalene46 have shown efficacy with a low incidence of adverse effects.47
For patients with more severe symptoms, isolated topical treatment may be time consuming and give suboptimal results, leading to the use of oral retinoids. The most widely used ones are acitretin, alitretinoin, and isotretinoin. Although they are particularly useful in diseases with thick scales such as lamellar ichthyosis, they have proven ineffective in purely erythrodermic forms and poorly tolerated by patients with skin fragility such as epidermolytic ichthyosis. Systemic retinoid-related side effects are usually dose-dependent and include cheilitis, nasal dryness, xerosis, hair loss, conjunctival irritation, and lipid and liver abnormalities. Chronic use may cause skeletal hyperostosis with spurs and calcifications of the spine and tendons. Additionally, they have a prolonged teratogenic potential with washout periods ranging from 1 month (isotretinoin and alitretinoin) up to 3 years (acitretin). Therefore, it is necessary to monitor patients undergoing treatment with oral retinoids, including liver enzymes, lipid profile, X-rays, and pregnancy tests. These considerations, along with age, determine the agent used, and the initial and maintenance doses that should be used.48–50
Pathogenesis-based therapiesRecently, therapies directed more specifically against the molecular alterations of EDD are beginning to appear.
One of these targets is immune dysregulation, since several EDD show polarization towards a Th17/IL-23 phenotype. Consequently, monoclonal antibodies directed against different proteins of this pathway such as IL-17A (secukinumab), IL-12 and IL-23 (ustekinumab) and IL4R/13R (dupilumab) are starting to be used. These antibodies have proven useful in some patients with desmosomal disorders51,52 or Netherton syndrome, but their irregular and/or transient efficacy does not allow generalized use.53
Replacement therapies are directed against the altered proteins and lipids in each EDD with the aim of supplying the altered molecules in each disease. Lipid replacement therapy has proven particular useful in CHILD syndrome where treatment with cholesterol and statins markedly improves the phenotype of most patients.54,55 Simultaneously, protein replacement therapy is being evaluated to deliver transglutaminase 1 in patients with lamellar ichthyosis.56
Gene therapy aims to supplement a healthy copy of the affected gene (pharmacological gene therapy) or repair the mutation in the patient's genomic DNA (curative gene therapy). To date, only pharmacological gene therapy strategies have been evaluated in clinical trials targeting TGM1 in lamellar ichthyosis57 and SPINK5 in Netherton syndrome.58 Curative gene therapy is in the early stages of development, due to how difficult it is to modify genomic DNA. Still, there is great interest as it would be the only treatment capable of curing genetic diseases.59
Special aspects of treatmentMany EDD present symptoms associated with skin barrier defects that also need to be controlled. Pruritus is very common and decreases quality of life significantly.60 Although treatment with monoclonal antibodies targeting inflammatory pathways (dupilumab,61 ustekinumab62 and secukinumab52) seems effective, results are inconsistent and often transient. Hypohidrosis increases the risk of heat stroke, making it necessary to limit physical exercise and stay in cool environments. In eye care, the main objective is to maintain lubrication via prophylactic use of artificial tears.63 Scaling in the ear canal can cause conductive hypoacusia, increase the risk of infections and cause irreversible damage to the eardrum, requiring regular otorhinolaryngological follow-up.64 In more severe forms, regular monitoring of metabolic parameters and adequate nutritional supplementation are required.65 The epidermal barrier defect facilitates the occurrence of bacterial and fungal infections, which may go unnoticed due to the cutaneous phenotype of EDD.
The extracutaneous symptoms of EDD highlight the critical need for multidisciplinary teams to provide adequate care. Ophthalmologists, otorhinolaryngologists, and nutritionists are especially important. Patients with extracutaneous signs need specialists according to the organs involved, such as neurologists, gastroenterologists, or traumatologists. For instance, many patients with recessive X-linked ichthyosis present attention deficit and hyperactivity disorders and need neuropsychological attention.66 Finally, some studies have shown that physical therapy can reduce the severity of symptoms and improve quality of life.67
Impact on quality of lifeEDD can have a profound impact on quality of life due to their physical manifestations, the need for constant skin care, and the associated social stigma. The visibility of the disease can lead to psychosocial difficulties and psychological problems, so both physical and emotional needs must be addressed.
Patient associations play a crucial role in improving quality of life by providing information, support, and resources. In Spain, the Spanish Ichthyosis Association (ASIC) (www.ictiosis.org) is dedicated to helping affected individuals and their families, promoting awareness, psychosocial support, and integration of patients into society. ASIC also promotes research and collaborates with health care professionals to improve disease management. This work is represented in Latin America by different associations depending on each country, such as www.ictiosis.cl in Chile, among others.
Conflict of interestÁ. Hernández-Martín is a researcher for Mayne Pharma and Celgene, a consultant/advisory board member for Sanofi, Viatris, Almirall, Galderma, and L’Oréal, and a speaker for Sanofi, Viatris, L’Oréal, Leti Pharma, Beiersdorf, and Alexion. The other authors declare no conflicts of interest.