A pathologic complete response rate of 49% has been observed in patients with unresectable melanoma treated with targeted therapy.1 Pathologic complete response is defined as the presence of less than 10% viable tumor cells after resection.
We report on 4 patients with unresectable metastatic melanoma who were able to undergo salvage surgery following good clinical and radiologic response to targeted therapy with dabrafenib plus trametinib. Histology showed a low number of viable tumor cells in all cases.
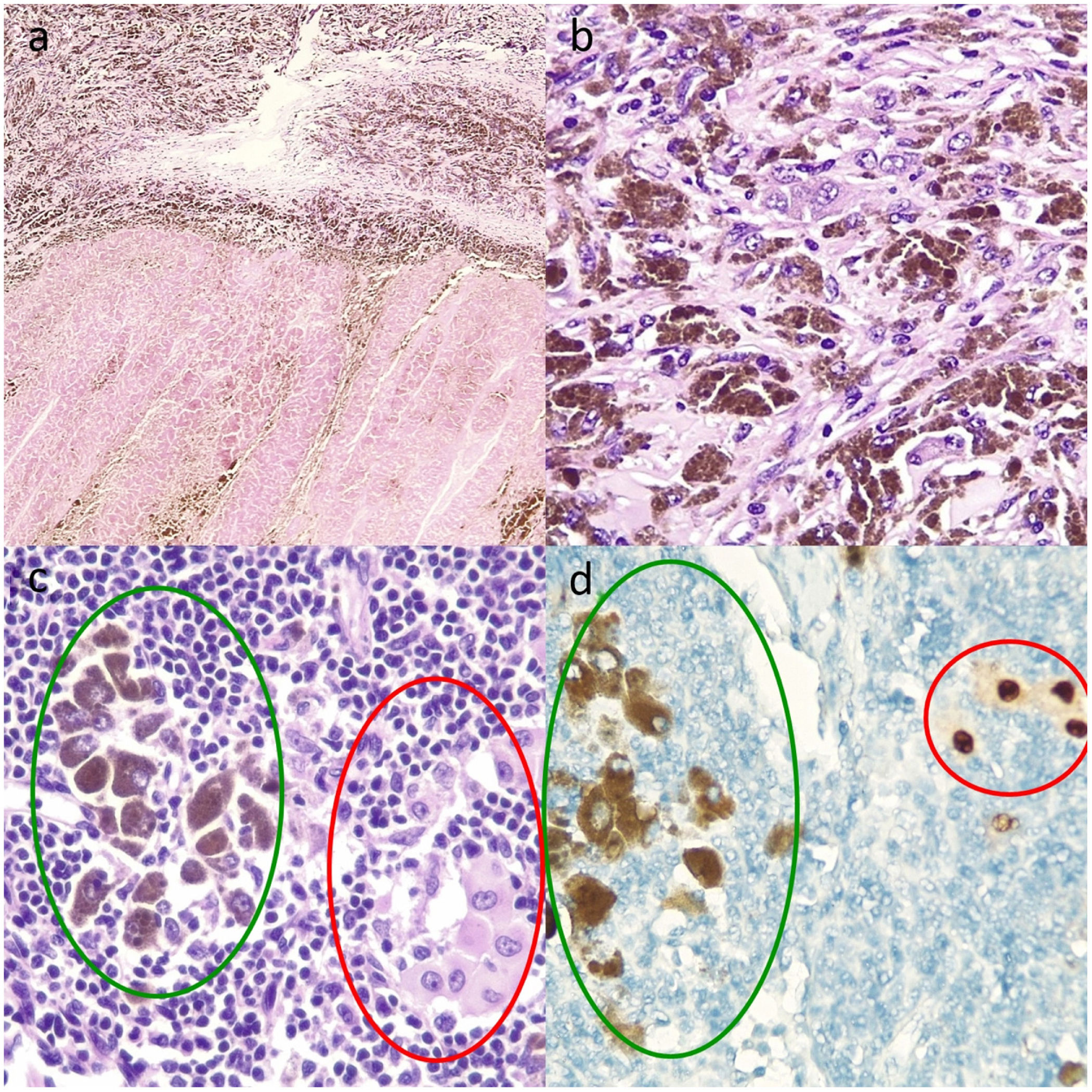
Four tumor specimens from patients with initially unresectable metastatic melanoma who underwent salvage surgery after achieving good clinical and radiologic results with dabrafenib plus trametinib were histologically examined. Radiologic and pathologic responses were evaluated using RECIST 1.1 (Response Evaluation Criteria in Solid Tumors)2 and the International Neoadjuvant Melanoma Consortium criteria,3 respectively. The histologic specimens were examined for fibrosis, necrosis, and viable tumor cells (immunohistochemical staining with SOX10) (Fig. 1). Pathologic response was considered complete when the percentage of viable tumor cells was less than 10% and partial when the percentage ranged between 10% and 50%.
Histologic sections of lymph nodes after targeted therapy with dabrafenib and trametinib. A, Multiple melanophages against a background of fibrosis (upper part of image) and intense necrosis (lower part) (hematoxylin and eosin, original magnification ×10). B, Melanophages and viable tumor cells with abundant intracytoplasmic melanin, whose presence requires the use of additional techniques, such as staining with the nuclear marker SOX10 to assess the percentage of viable tumor cells and accordingly pathologic response (hematoxylin–eosin, original magnification ×20). C, Melanophages with intracytoplasmic melanin (green oval) and isolated viable tumor cells (red oval) (hematoxylin–eosin, original magnification ×100). D, Nuclear staining of viable tumor cells (red circle) and cytoplasmic staining of melanophages with no nuclear expression (green oval) (immunohistochemical staining with SOX10, original magnification ×100).
Targeted therapy with dabrafenib plus trametinib resulted in a favorable clinical response (shrinkage or disappearance of metastases) in all 4 patients (Table 1). The 3 patients evaluated using RECIST 1.1 had a partial radiologic response. Pathologic response was complete in 3 patients and partial in 1. One woman experienced grade III asthenia and loss of appetite during therapy. These effects were resolved by a dose reduction, followed by incremental increases over 8 weeks and reintroduction of the full dose, which has been well tolerated to date. At the time of writing, all patients are free of recurrence.
Characteristics of Patients With Unresectable Metastatic Melanoma Tumors Treated With Dabrafenib and Trametinib.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age, y | 74 | 50 | 42 | 68 |
| Sex | Female | Male | Male | Female |
| Stage | Unresectable stage IIIC (T3bN3bM0) | Unresectable stage IIIC (T4aN1aM0) | Unresectable stage IIID (T4bN3cM0) | Unresectable stage IIIC (T2aN3cM0) |
| Previous treatment | No | Pembrolizumab (progression during adjuvant treatment) | No | No |
| Melanoma metastasis | Right cervical, supraclavicular, axillary, and retropectoral lymph nodes | Large left axillary lymph node mass | Large left axillary lymph node mass | Multiple cutaneous tumors on right leg |
| Systemic therapy used | Dabrafenib plus trametinib | Dabrafenib plus trametinib | Dabrafenib plus trametinib | Dabrafenib plus trametinib |
| Treatment time, wk | 16 | 8 | 6 | 48 |
| Clinical response | Disappearance of palpable lymph nodes palpables | Reduction in size of lymph nodes | Reduction in size of lymph nodes | Resolution of cutaneous lesions |
| Radiologic response | RECIST 1.1., partial | RECIST 1.1., partial | RECIST 1.1., partial | Not evaluated |
| Salvage surgery | Dissection of right cervical and axillary lymph nodes | Left axillary lymph node dissection | Left axillary lymph node dissection | Excision of residual pigmented cutaneous lesion |
| Pathologic response | Complete | Complete | Partial | Complete |
| Subsequent adjuvant treatment | Pembrolizumab with progression to cutaneous metastasisSwitch to dabrafenib plus trametinib | Dabrafenib plus trametinib | Pembrolizumab | Dabrafenib plus trametinib |
| Toxicity | Asthenia and loss of appetite (grade III), resolved by dose reduction | No | No | No |
| Disease-free survival, mo | 16 | 9 | 14 | 8 |
Abbreviation: RECIST, Response Evaluation Criteria in Solid Tumors.
Several adjuvant therapies have been approved for melanoma in recent years. Their use has improved disease-free survival in patients with resected stage III disease4–6 and triggered investigation into their potential in neoadjuvant settings, paving the way for what is now a dynamic research field.7 The use of neoadjuvant targeted or cytotoxic therapy is well established in the treatment of many types of cancer. It facilitates surgery, enabling complete tumor resection, and is also useful for identifying response biomarkers in resected specimens. A recent phase II randomized clinical trial compared the safety and efficacy of targeted therapy with neoadjuvant and adjuvant dabrafenib plus trametinib with standard surgical treatment in patients with resectable stage III or oligometastatic stage IV melanoma.8 The researchers observed a pathologic complete response rate of 58% and a 60% reduction in the risk of recurrence in patients who had received neoadjuvant therapy. In another phase II trial involving patients with stage III or oligometastatic melanoma, 49% of patients treated with neoadjuvant dabrafenib plus trametinib achieved a pathologic complete response.1 The corresponding rate in melanoma patients treated with the immune checkpoint inhibitor anti-PD1 as monotherapy was 21%.9
The current standard of care for resectable stage III and IV metastatic melanoma is surgery followed by immunotherapy or targeted therapy.10 Patients with initially unresectable metastatic melanoma, however, may be able to undergo salvage surgery if systemic therapy produces a radiologic response. Clinically, the 4 patients in the current series responded well to dabrafenib plus trametinib, as their metastases either shrank or disappeared completely. Three of the patients obtained a complete pathologic response, while 1 obtained a partial response. They all remain free of disease after a mean follow-up of 17.5 months (range, 10–24 months).
Thorough, standardized histologic examination of resected tumor specimens, with evaluation of necrosis, fibrosis, viable tumor cells, and melanophages, is essential for assessing treatment response. Additional tests may be necessary to distinguish between viable tumor cells and melanophages when intracytoplasmic melanin is present. Nuclear markers such as SOX10 are important for identifying viable tumor cells and evaluating pathologic complete responses.
Dabrafenib plus trametinib can potentially shrink unresectable BRAF-mutated metastatic melanoma, enabling subsequent surgery and reducing the risk of morbidity. Its use also provides a window of opportunity for evaluating the antitumor efficacy of targeted therapy via histologic examination of resection specimens. While persistent disease following treatment with dabrafenib plus trametinib may indicate the need to consider other adjuvant therapies, tumor eradiation may enable continuation of current treatment. Finally, neoadjuvant dabrafenib plus trametinib can potentially eradicate clinically occult disease, thereby delaying or even preventing subsequent recurrence.
Conflicts of InterestsThe authors declare that they have no conflict of interest.