Hair and scalp evaluation techniques can be classified into 3 categories: noninvasive methods (clinical history, general examination, inspection and palpation of the hair and scalp, photography, dermoscopy, etc.); semi-invasive methods (the trichogram); and invasive methods (biopsy). In this article, we provide a practical guide on how to evaluate hair and scalp conditions in the dermatology office.
Los métodos de exploración en tricología se pueden clasificar en 3 categorías: métodos no invasivos (historia clínica, exploración general, inspección y palpación del cabello y cuero cabelludo, fotografía, dermoscopia, etc.), métodos semi-invasivos (tricograma) e invasivos (biopsia). En este artículo se repasa de forma práctica el abordaje de los motivos de consulta en tricología en la consulta del dermatólogo.
Hair and scalp examination has earned a deserved place in dermatology for several reasons. First, hair consultations are very common in daily practice. An estimated 17.5% of all dermatology consultations are for hair loss and are more common than cosmetic, acne, or pediatric dermatology consultations. In fact, hair consultations account for 12.1% of all private dermatology practice revenue.1 Second, patients with hair problems are generally very worried about their condition and demand specialist care; in many cases they have already sought help in other specialized or nonspecialized centers. Finally, hair and scalp examination is a component of dermatology training for residents and also forms part of the portfolio of services offered by dermatology departments. It is therefore our duty to be familiar with the techniques involved. In this article, we provide a practical guide for evaluating hair problems in the dermatology office. This guide is based on our experience and supported by reports from the literature where possible.
Hair and scalp examination techniques can be divided into 3 categories: noninvasive techniques (history taking, general examination, inspection and palpation of the hair and scalp, photography, dermoscopy, etc.), semi-invasive techniques (trichogram), and invasive techniques (biopsy).2
Medical HistoryInvestigation of the patient's medical background should start with standard history-taking questions: “What brings you here today?” “How long have you had this problem?”, and “What do you think is the cause?” The answers to the first question (“What brings you here today?”) will tell us if the patient has a problem with too much hair (hypertrichosis or hirsutism), too little hair (alopecia, hypotrichia, or atrichia), or abnormal or dystrophic hair (irregular shape or growth). The second question (“How long have you had this problem?”) will help us to determine whether the disorder is congenital or intrinsic, whether it was present at birth or appeared later, or whether it was caused by external factors. Finally, the third question “What do you think is the cause?” will help us to discover the root of the problem in most cases.
Patients should be asked about the natural history of their condition: how it started, what changes they have noticed, if they have received previous treatments and if so, who prescribed them. In a recent study, Moreno et al.3 reported that 9% of patients with hair loss consult their hairdressers about the problem, compared with just 3% who consult a dermatologist. Learning about the natural history of the patient's condition will give us information about their understanding of the condition, as well as their level of concern and adherence to treatment. It is also important to ask about the use of cosmetic hair care products and to determine who applies them. Most cases of hair loss are due to changes in the hair cycle rather than to hair shaft defects, whereas it is the hair shaft that is affected, either favorably or adversely, by hair products. The inadequate, deficient, or aggressive use of such products can alter the quality and appearance of the hair, giving rise to acquired hair shaft anomalies (e.g., bubble hair).
Patients should also be questioned about family history, as this can reveal useful information in the case of androgenetic alopecia (AGA), which has been shown to have a polygenic pattern of inheritance. Family history is also of interest when a recessive or dominant hereditary condition is suspected. A personal past history of chronic systemic disease, a concomitant acute generalized disease, and the use of certain drugs may all be associated with effluviums. It is also important to rule out disorders of iron metabolism (particularly in women of childbearing age), thyroid disorders, low-calorie and low-protein diets, pregnancy, menopausal disorders, cancer, and, particularly, chemotherapy.
General ExaminationSigns of systemic disease, anemia, or thyroid disorders should all be investigated in a patient with effluvium.
If AGA is suspected in a female patient, the examination should focus on androgen-dependent signs. There are 3 systemic signs of androgen-dependent conditions in women. In Spanish, they are known by the triple M:
- -
Irregular Menstruation, with cycles of less than 21 days or more than 35 days, or episodes of amenorrhea lasting for more than 3 months in the previous 2 years.
- -
A body Mass index of over 25.
- -
Signs of Masculinization, with increased muscle mass, large genitalia, and changes in the tone of voice.
Androgen-dependent signs in the pilosebaceous unit are Seborrhea, Acne, Hirsutism, and Alopecia (SAHA syndrome).
Hair ExaminationThe hair should be examined using the techniques described below in the order shown4:
Noninvasive Techniques- 1.
Inspection: color, pattern of hair loss, hair density
- 2.
Palpation: Jaquet's sign, Sabouraud's sign, pull sign
- 3.
Instrument-aided examination:
The wash test (Rebora method): to assess hair loss
The hair growth window: to assess hair growth
Digital photography: to assess changes over time
Sebumetry: to assess sebum production
Trichoscopy
Histological studies are reserved for very specific cases, for research, and for scarring alopecia.
InspectionHair ColorHair color has no particular function. The more eumelanin in the cortex, the darker the hair will be and the more pheomelanin, the redder it will be. Hair color also provides information about the patient's skin phototype. Color changes may be due to inflammatory conditions that affect the hair follicle. An example is alopecia areata, where bald patches are commonly repopulated by finer, white hair. Other causes of color change are physical treatments (ionizing radiation, radiation therapy), the use of cosmetic products, and emotional stress.
Hair Loss PatternsHair loss is classified as diffuse, circumscribed, or localized, depending on the form of presentation.
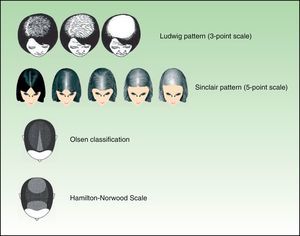
Diffuse and Progressive Hair Loss: AGA5Male-pattern hair loss, or AGA, is more common in men, but it can also affect women. It is characterized by a receding hairline in the frontal and vertex regions. With time, the balding areas merge. Two systems are used to classify AGA: the Hamilton-Norwood Scale, which describes 7 stages, and the Ebling system, which describes 5 stages. Female-pattern hair loss, which can also affect both men and women, is characterized by diffuse, progressive hair loss in the central region, with sparing of the front hairline. It is classified using the 3-point Ludwig Scale or the 5-point Sinclair Scale. Another pattern, described by Olsen,6 is diffuse thinning in the central area with more accentuated loss in the interparietal region, forming a triangular pattern (Fig. 1).
Sudden Diffuse Hair Loss: EffluviumsSudden diffuse hair loss typically has a systemic origin. In anagen effluvium, shedding occurs immediately after exposure to an external trigger while in telogen effluvium, it starts after about 2½ to 4 months. In both cases, it affects the parietal and frontal hairlines to a greater or lesser degree. Recovery is the rule and occurs on disappearance of the cause.
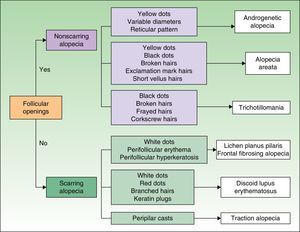
Patchy Hair LossAlopecia areata, tinea capitis, trichotillomania, and scarring alopecia should be included in the differential diagnosis in patients with patchy hair loss.
In alopecia areata, the alopecic patch tends to have black dots, broken hairs, and exclamation mark hairs (which indicate that the disease is active and that the patch is advancing), or fine hairs (which are signs of regrowth). In tinea capitis, it is common to find scaling on the surface of the scalp, corkscrew hairs, and progressive enlargement of the patch. In trichotillomania, the patch tends to have short hairs and/or hairs of different lengths (regrowth after pulling out); patients tend to be indifferent, even in cases of extensive hair loss. In scarring alopecia, the patch is completely bald, with a smooth shiny appearance and no hair follicles.
Marginal Hair LossThe differential diagnosis in patients with marginal hair loss should include traction hair loss, congenital triangular alopecia, ophiasis, sisaipho, lichen planus, and frontal fibrosing alopecia.
Traction hair loss is an acquired condition and most often affects the parietal region, unlike congenital triangular alopecia. Ophiasis is a form of alopecia areata that affects the frontal, parietal, and temporal margins of the scalp, and sisaipho is the inverse form (i.e., with sparing of these margins). Both lichen planus and frontal fibrosing alopecia are forms of scarring alopecia. Frontal fibrosing alopecia was initially described in postmenopausal women with hair loss affecting the frontoparietal hairline, but there have since been reports of shedding as far as the temporal and occipital regions, as well as reports in men and premenopausal women.
Hair DensityHair density depends on the number of hairs on a person's head and the diameter of each hair. The diameter ranges between 57 and 90μm in Europeans and can be as high as 120μm in Asians. The number of hairs a person has varies with age, from 1100 follicles/cm2 in children to 600 follicles/cm2 in a 25-year-old adult, and just 300 follicles/cm2 in an adult aged between 30 and 50 years. Hair loss is thus also physiological, with hair becoming less dense as people age.
Other Signs on Visual InspectionInspection of the hair can also reveal signs indicating the possible involvement of the scalp in inflammatory conditions such as psoriasis or seborrheic dermatitis. There may also be scars on the scalp from previous surgery, radiation therapy, tumors, etc.
PalpationThe Fold Sign or Jacquet's SignThe fold sign test consists of folding an area of the scalp between the 2 thumbs. If several folds are formed easily, the test is positive and indicates an absence of hair fibers in some, many, or all of the follicles, the presence of miniaturized hair (AGA), or the absence of follicles (scarring alopecia).
Sabouraud's SignThe Sabouraud sign is elicited after removing a group of hairs for trichogram using a rubber-sheathed Kocher forceps. Increasing traction is applied to the hair while it is still held between the blades of the forceps. The test can also be performed by holding a tuft of hairs firmly between 2 fingers of one hand and pulling with increasing force on the free ends of the hairs with the other hand. It is used to measure resistance of the hair to traction and is positive if the hair breaks easily. Positive results are mainly seen in patients with alterations of the hair shaft or hair damaged by external aggression.
Pull TestThe pull test is performed by tugging firmly on a group of 20 to 50 hairs. It is positive if a large number of hairs are pulled away. The results are not very useful if the patient washes his/her hair before the test, as any hairs that are about to be shed will fall out during washing. This test is positive in anagen and telogen effluviums. Microscopic examination of the root ends of the hairs will help to distinguish between the 2 conditions.
Instrument-Aided InspectionHair Growth WindowA 1-cm2 area of hair is shaven or clipped as close to the scalp as possible using a razor or thin scissors and a perforated ruler. The length of the hair that regrows in this area is measured after a week. The normal hair growth rate is 2.5mm a week (1cm a month). This test is used to measure hair growth, and more importantly, to convince patients that their hair is growing well. It is not necessary in patients who dye their hair regularly as the same information can be obtained by measuring the distance between the roots and the point at which the color changes.
The Wash Test (Rebora Method)In this test, the patient washes his/her hair after 5 days of not washing it and collects any hair shed in a gauze placed over the hole in the washbasin. The patient then takes the hairs to the dermatology office, where they are classified according to length: >5cm, 3-5cm, or <3cm. Long and short hairs are then placed on appropriately sized glass slides for microscopic examination. The aim of this examination is to determine the state of the root and measure the diameter of the hairs. Results are interpreted on the basis that normal hair grows at a rate of 1cm a month.
A predominance of hairs shorter than 3cm and the presence of over 200 hairs is suggestive of a diagnosis of chronic telogen effluvium. In this disorder, the anagen phase is shorter than normal and hair is shed in the telogen phase. The diagnosis will be AGA when over 10% of the hairs shorter than 3cm are miniaturized, as hair miniaturization is progressive in this disorder. The anagen phase in AGA becomes progressively shorter, leading to increasingly shorter and finer hair. The suspected diagnosis in a patient in whom hairs longer than 5cm predominate will be acute telogen effluvium. Hair grows well in this condition, and is generally longer than 5cm when, for whatever reason, it enters the shedding phase.
Digital PhotographyThe principles of digital photography of the hair are the same as those that apply to the skin. Lighting is very important as excessive light can give the impression that there is less hair than there really is. Likewise, insufficient lighting will make it seem as if there is more hair. Digital photography in hair disorders is mostly used to evaluate changes in presentation and response to treatment. The photographs should therefore always be taken using consistent lighting, camera-to-patient distance, patient position, and hair style. Digital photography is also used for trichoscopy (dermoscopy applied to the hair and scalp) and the trichogram.7
Sebumetry: Measuring Sebum ProductionSebumetry is a technique that involves the photometric analysis of sebum secreted by the scalp. The sebum is collected using a special tape whose transparency changes according to the amount of sebum. The results are read on a scale of 1 to 100 and provide an objective measurement of sebum production for initial evaluation and monitoring of treatment.
TrichoscopyTrichoscopy is used as an adjunct in the clinical examination. It involves using a dermoscope to examine the surface of the scalp and the hair shaft to identify signs of scalp disorders and different types of alopecia.8,9 Key trichoscopic findings are shown in Figure 2.
Semi-Invasive Microscopic Techniques: The TrichogramThe trichogram was first described in the literature in 1964.10,11
It is a simple, minimally invasive, rapid and economic technique for measuring hair follicle activity. It involves the microscopic examination of hairs plucked from the scalp and provides information about the state of the proximal end of the hair shaft (the root) and the distal end (the tip). The trichogram is a useful complementary tool for clinical evaluation, diagnosis, and the monitoring of treatment response.
It should be noted that the trichogram simply provides a snapshot of the hair follicle at the time of examination, and that the condition of follicles can vary within the same patient depending on numerous factors, such as sampling site, previous washing or brushing of the hair, and time of the year.12
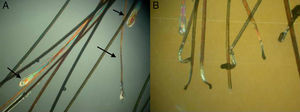
Sample Collection and PreparationCorrect sample collection and preparation is very important. The technique used will vary depending on the purpose of the trichogram (Fig. 3):
- 1.
To examine the proximal end of the hair shaft, the first step is to select an appropriate sampling site. In male-pattern hair loss, the first sample should be taken from the central interparietal area, while the second sample, if needed, should be taken from the temporal or occipital area. In female-pattern hair loss, samples should be taken from the center and the vertex of the scalp. The sites for telogen hair loss and scarring alopecia are, respectively, the central interparietal area and the advancing border of the alopecic patch.
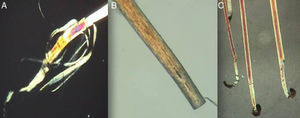
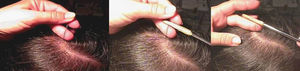
Using a rubber-sheathed Kocher forceps, remove a tuft of 15 to 20 hairs. To do this, place the forceps 1 to 2cm from the scalp and pluck out the hairs rapidly and firmly in the direction of the natural growth of the hair. If the hairs are not plucked out firmly, they may appear as pseudo-dystrophic hairs under the microscope, or exhibit frayed or broken roots (Fig. 4).
In a patient with short hair, epilation forceps with rubber-sheathed tips should be used to gently pull out the hairs so as to not modify the characteristics of the hair shaft.
A bladder catheter of a suitable size can be used to cover each blade of the forceps.
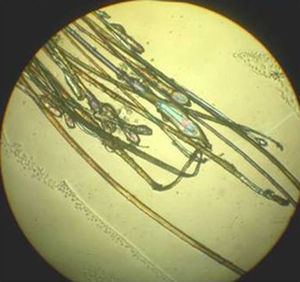
The next step is to prepare the hairs for examination under the microscope. They should be carefully placed on a glass slide, ensuring that they are parallel to each other and that the roots are aligned. Next, they are covered with clear adhesive tape. This is the simplest method but the use of adhesive tape can produce artifacts, such as bubbles and black spots, that distort the image. To avoid these artifacts and obtain a sharper, cleaner image, apply several drops of balsam (such as that used to mount histological slides), and cover the hairs with a cover slip. The use of polarized light improves image quality.
- 2.
To examine the hair shaft, place the slide, prepared as above, under the microscope and examine the length of the shaft and the root. In the case of hair dystrophy, where the shaft needs to be examined in greater detail, use scissors to cut the hairs at the level of the scalp and place them on a long glass slide. Avoid any traction on the hairs.
- 3.
To examine the distal end of the hair shaft, in the case of long hair, cut the hairs close to the distal end of the shaft and in the case of short hairs, pluck them out using epilation forceps.
The sample is examined using a 4x objective, although a 10x or 40x objective can be used if higher magnification is needed. A higher-quality image can be obtained by fitting 2 polarizers to the microscope: 1 between the condenser and the sample and the other between the sample and the observer.
The different parts of the hair shaft should be analyzed in the trichogram.
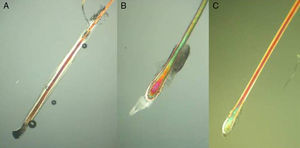
The Proximal EndExamination of the proximal end of the hair shaft helps to determine whether a hair is in anagen, telogen, or catagen phase (Table 1, Fig. 5), and whether it is normal or dystrophic.
Anagen hair shafts are longer, have a uniform diameter, a rectangular shape, and a slight distal angle. Pigmentation is intense in the bulb area and there are sheaths and membranes.
Telogen hair shafts are shorter and appear higher up in the trichogram, above the roots of anagen hairs; the root is thick and club shaped and there are no distal angles. Pigmentation is weak or absent; the sheath is also absent or found only at the distal end.
Observation of Proximal End of Hair Shaft. Characteristics of Anagen, Telogen, and Dystrophic Hairs.
| Anagen | Telogen | Dystrophic Hair |
| Longer | Shorter | |
| Uniform diameterRectangular shape | Thickened root (club shaped) | Tapering diameter: exclamation markIrregular contour |
| Intense pigmentation | Weak pigmentation | |
| Presence of sheaths | Sheath at root only or absent | No sheaths |
| Slight distal angle (<20°) | No angles | Distal angle >20° |
Proximal end of the hair shaft. A, Anagen hair. B, Catagen hair. C, Telogen hair. See Table 2 for characteristics. Polarized light microscope (original magnification ×40).
Very few hairs in the catagen phase are observed in the trichogram as they account for a very small percentage of all hair.
The anagen to telogen ratio varies, mainly according to age and sex. Children have the highest percentage of anagen hair (95% anagen vs 5% telogen), and the ratio decreases with age. The anagen to telogen ratio is 86:11 in women and 83:15 in men. In a normal trichogram, an average of 89% of hairs are in anagen, 10% in telogen, and 1% in catagen. A diagnosis of telogen effluvium is established when over 20% of the hairs examined are in telogen phase.
Dystrophic hairs have a decreased proximal diameter, an irregular contour, no epithelial sheaths, and an angle of over 20°. They are common in AGA or in hair that has not been removed correctly from the scalp.
Keratotic material may be observed on the tip of the hair in conditions such as seborrheic dermatitis, psoriasis, and folliculitis. A common finding in patients with demodicosis is the presence of Demodicosis folliculorum in contact with the root of the hair, although this condition is usually diagnosed by superficial skin biopsy.
Three aspects of the hair shaft are examined.
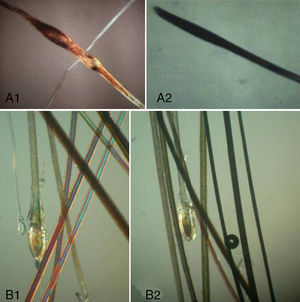
UniformityNormal hair is uniform in appearance and structure along the entire length of the hair shaft; this uniformity is also observed between the different hairs in a sample (Fig. 6).
Uniformity within the same hair shaft. Hair dysplasias are malformations of the hair shaft. Although scanning electron microscopy is the diagnostic tool of choice in such cases, certain signs may be observed in the trichogram13:
- •
Monilethrix (beaded hair): Alternating segments of narrowings and nodosities, giving a characteristic beaded appearance.
- •
Pseudomonilethrix: Round hairs with irregular, sporadic rounded nodosities. There are no narrowings.
- •
Pili torti: Twists of hairs with bending at different angles and regular intervals.
- •
Trichorrhexis invaginata (bamboo hair): Ball-shaped deformity with cupping at the proximal end of the hair shaft.
- •
Trichothiodystrophic hair: Patients with trichothiodystrophy may have hair with ribbon-like flattening, characteristic trichoschisis-like fractures (clean transverse breaks), with an irregular surface and tiger-tail banding.
- •
Trichonodosis: Single or double knots on the hair shaft. Tie knots and other more complex knots are also observed.
- •
Trichorrhexis nodosa: Bulging hair characterized by fracture nodes with open splitting of the cortex on both sides of the node. If the hair eventually splits, it will leave a brush-like appearance at both ends.
- •
Bubble hair: Short, broken hairs with a wavy surface and bubbles inside the shaft.
- •
Loose anagen hair: Twisted anagen hairs with a ruffled cuticle at the proximal end. Long, pili canaliculi–type canals on the shaft are a common finding.14
- •
Pili annulati: Hair shafts with alternating light and dark bands.
- •
Woolly hair: Thin curly hair forming small woolly balls.
- •
Uncombable hair (pili canaliculi): Canalicular formation along the entire length of the hair shaft. This formation can be difficult to spot under a microscope but the micrometer can be moved if pili canaliculi is suspected.
In alopecia areata, the attack launched by the immune system arrests the anagen phase of the hair, but this eventually returns to normal growth. The result is a hair shaft with alternating narrow and normal sections. In some cases, the diameter can be so narrow that it causes transverse fracture of the hair shaft. Pseudomonilethrix and/or trichoschisis may be observed in some hairs.
Uniformity between hair shafts. Under normal conditions, all the hair shafts in a sample should have a similar diameter. The detection of hairs with different diameters should raise a suspicion of AGA. In this condition, the anagen phase becomes progressively shorter. Consequently, hairs enter the anagen phase more often, leading to more frequent shedding of increasingly shorter hairs. The diameter also decreases, with shafts becoming progressively narrower. Hair shaft diameter variability has been demonstrated in women, with larger diameters seen in higher stages of the Ludwig Scale15; the differences were minimal at stage I and maximal at stage III.
External AggressionExternal aggression can give rise to cuticle abnormalities. The most common causes are the use of cosmetic products or intense sun exposure. The diagnostic tool of choice in such cases is scanning electron microscopy.
Substance DepositsDry dandruff or scales in patients with seborrheic dermatitis or psoriasis commonly become attached to the hair shaft, where they form sleeve-like structures that encircle the hair (peripilar casts). Deposits may also be seen on the scalp and hair of patients who use topical formulations of minoxidil in which the particles remain in suspension due to poor compounding. Nits or lice may be seen in patients with pediculosis capitis (Fig. 7).
The Distal EndTo examine the distal end, hairs should ideally be removed with a scissors. If forceps are used, the blades should be fitted with a protective sheath and the hair plucked gently to maintain the structures intact.
Three types of hair shaft tips can be observed (Fig. 8):
- 1.
Javelin tip: A very sharp, spear-like tip is seen in hair that is growing well and has never been cut.
- 2.
Paintbrush tip: Fractures in the hair shaft (trichoschisis) give the tip a paintbrush-like appearance (Fig. 8), seen in hair shaft anomalies such as monilethrix, alopecia areata, or in hair fragility induced by cosmetic products. The shaft has a frayed, ruffled tip.
- 3.
Clean-cut tip: The distal end has been cut and ends in a perfectly straight line. It is typically seen in hair that has been cut and in cases of trichotillomania (Fig. 8B).
AGA is characterized by an increased proportion of telogen hairs, the presence of dystrophic hairs, and heterogeneity between hair shafts, which are of variable thicknesses (Table 2, Fig. 9, A and B).
Hair Loss Patterns. Clinical Examination and Trichogram.
| Clinical Examination | Trichogram | |
| Androgenetic Alopecia | Fold test ±Less density of hair(Ludwig/Ebling) | Higher proportion of telogen hairsDifferent diametersSome dystrophic hairs |
| Alopecia Areata | Pull test+ if activityFold test+Exclamation mark hairsBlack dots | Hair shafts with narrowingsFracturesPaintbrush tip |
| Anagen effluvium | Pull test+++Immediate cause | Predominance of anagen hairs (golf club–shaped proximal end) |
| Telogen effluvium | Pull test++Cause 3-4 months earlier | >20% hairs in telogen phase (club-shaped distal end) |
| Trichotillomania | Many unresolved questions in patient history and clinical examination | Cut hairs (clean-cut distal end) |
| Hair fragility | Altered shaftTraction test++ | Hair shaft alterations (seen better with scanning electron microscopy) |
In alopecia areata, the examination of exclamation mark hairs (plucked gently using rubber-sheathed forceps) shows a shaft with alternating narrow and normal segments and a distal end with a paintbrush appearance resulting from fracture.
Telogen EffluviumMore than 20% of hairs in telogen effluvium are in telogen. The hairs are shorter than normal, have a uniform diameter, a rounded proximal end (club-like appearance) and a lack of pigment and membranes (Fig. 10).
Anagen EffluviumHair loss is alarming in anagen effluvium. The pull test results are strongly positive and normal anagen hairs are seen in the trichogram. The hairs are longer than telogen hairs, are pigmented, and have sheaths and membranes. The distal end is angled like a golf club.
TrichotillomaniaIf the patient's history and examination indicate a possible diagnosis of trichotillomania, several hairs should be taken from an affected and an unaffected area. The hairs in the unaffected area will exhibit signs of normal growth, while those in the affected area will have a normal proximal end (in anagen or telogen), a hair shaft with alterations that vary according to how the hair has been treated, and a clean-cut distal end (Fig. 8B).
Ethical DisclosuresProtection of humans and animals. The authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Serrano-Falcón C, Fernández-Pugnaire MA, Serrano-Ortega S. Evaluación del pelo y cuero cabelludo: tricograma. Actas Dermosifiliogr. 2013;104:867–876.