Although only a small area of the body is affected in palmoplantar psoriasis or psoriasis of the palms and soles, this condition causes considerable functional impairment due to hyperkeratosis, fissures, and erythema, and occasionally inflammation and pustules. These symptoms may seriously interfere with the patient's quality of life and may be disabling.1 Moreover, the lack of a standard treatment hinders the therapeutic management of this clinical variant. We report a case of hyperkeratotic palmoplantar psoriasis that had not responded to several conventional therapies but responded well to treatment with ustekinumab.
The patient was a 56-year-old man with a 1.5-year history of palmoplantar psoriasis whose treatment history at another hospital was as follows: high potency topical corticosteroids and calcipotriol (no improvement); topical psoralen UV-A therapy 3 times a week for 6 months, (poor response); and methotrexate 15mg/wk associated with elevated transaminase values (5 times baseline) and marked gastrointestinal symptoms that led to withdrawal of treatment after 2 months.
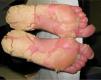
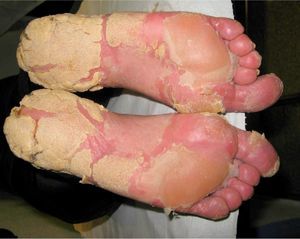
The patient presented at our hospital with severe palmoplantar hyperkeratosis, fissuring, and 100% involvement of the palms and soles; it was difficult for him to walk and carry out his daily activities (Fig. 1). There were no other lesions or joint involvement. Treatment with acitretin 50mg/d (weight 76kg, 0.66mg/kg) resulted in some improvement, but was poorly tolerated because of dry skin, cheilitis, joint pain, gynecomastia, alopecia, and hypertriglyceridemia (352mg/dL). Reduction of the dose to 35mg yielded little improvement in the side effects and worsening of the lesions. Consequently, treatment was suspended after 9 months.
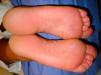
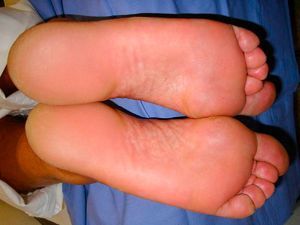
Owing to the failure of conventional therapies, we decided to begin treatment with a biologic agent. As the patient was a frequent traveler who spent long periods away from home, ustekinumab 45mg was prescribed and administered according to the conventional regimen (initial dose followed by another 4 weeks later and every 3 months thereafter). We successfully applied for a compassionate use permit and the patient duly signed informed consent. At 16 weeks, after receiving 2 doses of ustekinumab 45mg, the patient reported complete resolution of his disease (Fig. 2). Clinical findings and tests showed no drug-related side effects. The patient has continued with the treatment for the last 12 months with excellent results and no adverse events.
Hyperkeratotic palmoplantar psoriasis has traditionally been treated with the drugs used for psoriasis vulgaris (with varying, but generally poor, results) and often represents a challenge to dermatologists.1–4 In cases where conventional therapies have failed, anti-tumor necrosis factor agents have proved effective.2,5 However, cases have also been reported in which these agents have paradoxically been associated with new onset or worsening of palmoplantar psoriasis or pustulosis. The mechanism is poorly understood and biologic drugs should therefore be used with caution in this setting.6 Efalizumab is another biologic agent that has proved to be effective in palmoplantar psoriasis,3 although international sales of this drug have now been discontinued because of an increased risk of progressive multifocal leukocytopenia.
Ustekinumab – the biologic agent most recently approved for use in psoriasis – is a fully human monoclonal antibody that binds to the shared p40 subunit of the interleukins (IL) 12 and IL-23 and blocks their action. To date, ustekinumab has only been studied in plaque psoriasis, and pustular, erythrodermic, and palmoplantar psoriasis are not, therefore, included in the summary of product characteristics; the only evidence available on its use in these variants comes from case series and isolated case reports. Since ustekinumab is a new drug, experience of its use in forms other than plaque psoriasis is scant. Daudén et al.7 reported a case of generalized pustular psoriasis that responded with excellent results following treatment with ustekinumab. In a series of 4 patients with palmoplantar pustulosis, Gerdes et al.8 reported good response in 1 patient, no response in 2 patients, and a partial response in the fourth. On the basis of these findings, they did not consider ustekinumab to be an appropriate treatment for regular use in this disease. Two other authors recently reported good results with ustekinumab in hyperkeratotic psoriasis in patients similar to ours.4,9 From a pathophysiologic standpoint, increased IL-23 expression has been observed not only in plaque psoriasis but also in palmoplantar psoriasis and hyperkeratotic hand dermatitis.10 Moreover, IL-23 stimulates production of IL-17 and IL-22, and the latter induces epidermal hyperplasia and acanthosis, key pathologic findings in psoriasis.11 Thus, the inhibitory effect of ustekinumab on IL-23 could explain the improvement of palmoplantar hyperkeratosis treated with ustekinumab. However, further studies involving larger numbers of patients are needed to determine whether ustekinumab is useful in this setting.
Conflicts of interestDr. Almudena Nuño González, Dr. Enrique Gómez de la Fuente, and Dr. Francisco Javier Vicente Martín declare that they have no conflicts of interest.
Dr. José Luis López Estebaranz has taken part in clinical trials and evaluations for Janssen, Abbott, Pfizer, and Schering-Plough.
Please cite this article as: Nuño-González A, et al. Psoriasis hiperqueratósica palmoplantar con excelente respuesta a ustekinumab. Actas Dermosifiliogr. 2012;103:169–70.