Fournier gangrene is a urological emergency associated with a high mortality. It is a necrotizing fasciitis caused by polymicrobial infection originating in the anorectal or genitourinary area. The aim of this study was to analyze the epidemiological and clinical characteristics of Fournier gangrene along with the variables that influence disease course and mortality in patients treated in our department.
Material and MethodsWe carried out a retrospective study of 37 patients diagnosed with Fournier gangrene between January 2001 and October 2010.
ResultsAll the patients were men, 43.2% had diabetes, and the mean age of the patients was 57.68 years. Statistically significant differences were observed between the age of surviving patients and that of patients who died (55.8 and 69.6 years, respectively). The mean hospital stay was 27.54 days and 32.4% of patients required admission to the intensive care unit. Etiology was unknown in 39.8% of cases. Polymicrobial infection was observed in 59.5% of cases. The mean health care cost associated with a patient diagnosed with Fournier gangrene admitted to intensive care and requiring at least 1 procedure in the operating room was €25108.67. Mortality was 13.5%. Based on analysis of individual comorbid conditions, only ischemic heart disease displayed a statistically significant association with mortality due to Fournier gangrene; ischemic heart disease was also associated with longer hospital stay.
ConclusionsFournier gangrene is associated with high mortality despite appropriate early treatment. Although the condition is infrequent, the high associated health care costs suggest that primary and secondary prevention measures should be implemented.
La gangrena de Fournier es una urgencia urológica definida como una fascitis necrotizante, con una alta mortalidad, resultado de una infección polimicrobiana que se origina en la región anorrectal y/o genitourinaria. El objetivo de este estudio es analizar las características epidemiológicas y clínicas, así como las variables que han influido en la evolución y mortalidad de los pacientes tratados en nuestro Servicio.
Material y métodosEl estudio analiza retrospectivamente 37 pacientes diagnosticados de gangrena de Fournier en el periodo de tiempo comprendido entre enero del 2001 a octubre de 2010.
ResultadosTodos los pacientes son hombres, con una edad media de 57,68 años, existiendo diferencias estadísticas en la edad de los fallecidos respecto a los que sobreviven, 69,6 años frente a 55,8 años. El 43,2% eran diabéticos. La estancia media hospitalaria fue de 27,54 días. El 32,4% precisó de ingreso en la UCI. En el 39,8% se desconoce su etiología. La infección fue polimicrobiana en el 59,5% de los casos. El coste sanitario medio de un paciente diagnosticado de gangrena de Fournier que ingresa en la Unidad de Cuidados Intensivos (UCI) y requiere de al menos una cura en quirófano es de 25.108,67 euros. La mortalidad fue del 13,5%. Al estratificar las patologías estudiadas de forma independiente se observa que sólo la cardiopatía isquémica se relacionó de forma significativa con la mortalidad y una mayor estancia hospitalaria.
ConclusiónLa gangrena de Fournier es una patología con una alta mortalidad, a pesar de un tratamiento adecuado precoz. Es una patología con una baja incidencia, pero supone un coste elevado para el sistema sanitario, por lo que serían necesarias medidas de prevención primaria y secundaria.
Fournier gangrene is a urological emergency that was first reported in 1764 by Baurienne,1 although it was not until 1883 that the French venereologist Jean Fournier described the clinical characteristics of the disease in a series of 5 cases with no apparent cause.2
Fournier gangrene is defined as necrotizing fasciitis resulting from a rapidly progressive polymicrobial infection involving aerobes and anaerobes acting synergistically. The disease originates in the anorectal and genitourinary areas and can reach the groin, legs, anterior wall of the abdomen, and even the thorax, given its ability to progress across the fasciae of Buck, Dartos, Colles, and Scarpa.3–5
Progression results from thrombosis of the small subcutaneous vessels secondary to endarteritis obliterans, which produces tissue hypoxia and limited vascular supply, thus facilitating overgrowth of anaerobic microorganisms and making it difficult for antibiotics to reach these areas.1,6
Although there have been reports in women and even in children as young as 2 months,7,8 the disease mainly affects men aged 50–70 years.9
The overall incidence of the disease is 1.6 cases per 100000 person-years,7 although mortality is high (20%–30%, on average,10 according to recent series), despite initiation of appropriate treatment, which consists of adequate hemodynamic stabilization, early and radical debridement, broad-spectrum antibiotic therapy, and daily wound care.
Many patients have underlying systemic diseases (e.g., diabetes mellitus, urogenital tuberculosis, syphilis, human immunodeficiency virus infection, cancer, and chronic alcoholism), which are responsible for the vascular and immune disorders that increase susceptibility to polymicrobial infection.5 Low socioeconomic level has also been reported to be a predisposing factor.11,12
Using data from the patients treated in our department, we analyzed the clinical and epidemiological characteristics of Fournier gangrene to compare them with the findings of previous reports. We also analyzed those variables that affected outcome and mortality.
Material and MethodsWe retrospectively analyzed 37 patients diagnosed with Fournier gangrene at Hospital Universitario San Cecilio in Granada, Spain between January 2001 and October 2010. The disease was coded according to the International Classification of Diseases, Ninth Revision as Fournier gangrene (728.86). Clinical diagnosis was based on the patient's medical history and physical examination, which included as diagnostic criteria the presence of foul-smelling necrotic slough in the anogenital area associated with crepitus in the context of sepsis.
The variables studied were as follows:
- 1.
Personal details: age and sex.
- 2.
Personal history, including mainly presence of diabetes mellitus, chronic alcoholism, obesity, perianal abscess or fistula, and urethral stricture.
- 3.
Presence or absence of previous multiple conditions. We defined multiple conditions as the presence of 2 or more chronic diseases that can affect normal performance of activities of daily living and require close follow-up by a clinician.
- 4.
Urinary catheterization before diagnosis of Fournier gangrene.
- 5.
Identification of causal agents (monomicrobial or polymicrobial).
- 6.
Need for reconstructive surgery: secondary suture, placement of skin grafts or flaps.
- 7.
Outcome (mortality attributable to infection).
- 8.
Admission to and length of stay in the intensive care unit (ICU).
- 9.
Length of hospital stay.
- 10.
Mean overall health care costs arising mainly from the hospital stay and the use of an operating room for wound care. The cost was calculated using Coan-HyD, the cost calculation program used by the Andalusian Health Service.13 We calculated the unit cost, which was defined as the total cost (direct costs+indirect costs) divided into product units (i.e., stay in the ICU, stay on the urology ward, and time [hours] in the operating room) for a stay in the ICU (€1609.65 per day), a stay on the urology ward (€373.82 per day), and hours of operating room time with 1 surgeon (€884.05 per hour). Each unit cost was multiplied by the mean stay in the ICU, the mean stay on the urology ward, and the mean time in the operating room.
Data were analyzed in a purpose-designed database using SPSS version 17.0 and by applying the χ2 test or the t test, as appropriate. Statistical significance was set at P≤.05.
ResultsWe analyzed 37 patients diagnosed with Fournier gangrene during the study period. Mean (SD) age was 57.68 (15.56) years. As for personal history, 21.6% of patients had chronic alcoholism, 43.2% had diabetes (insulin-dependent or not), and 24.3% had had some degree of ischemic heart disease. Local involvement of the genital and perineal areas was as follows: urethral stricture, 16.2%; perianal fistula or abscess, 29.7%; furuncle, 15.3%; no local involvement before diagnosis, 39.8%. Multiple conditions were recorded in 32.4% of patients. None of the patients analyzed had a urinary catheter before diagnosis.
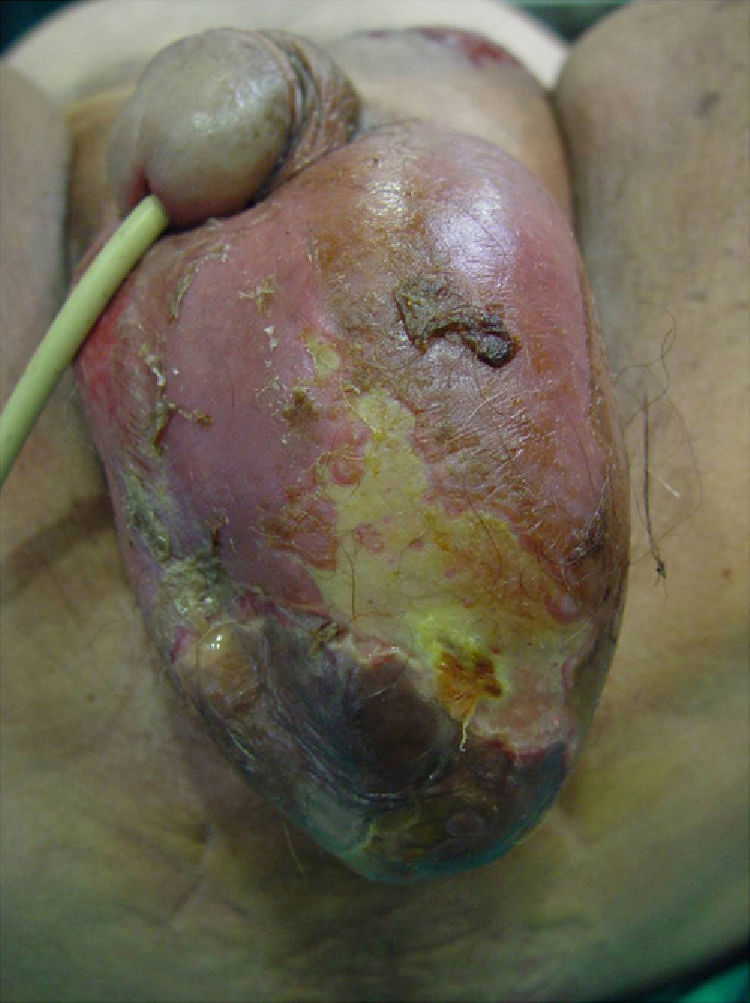
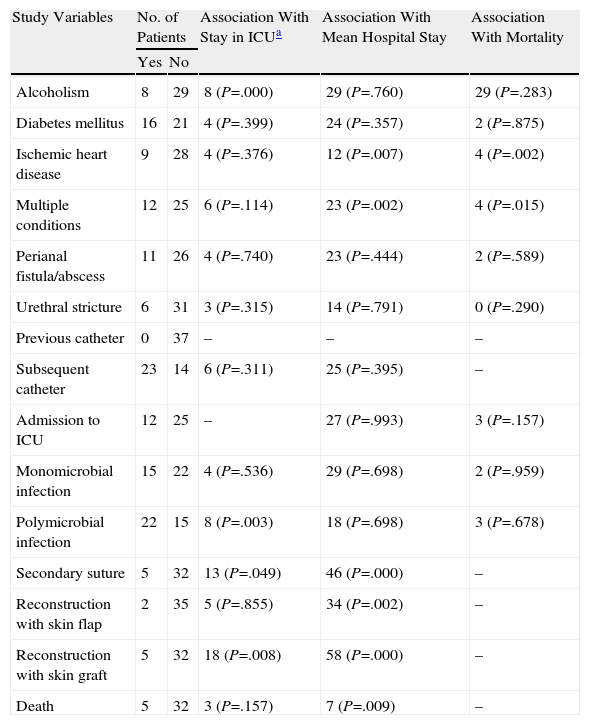
The sites involved on admission were as follows: scrotum, 54.05%; perineum, 37.83%; perianal area, 30%; penis, 27.02%; suprapubic area, 16%; and hypogastrium, 5.4%. All patients had some degree of edema on the penis or scrotum, 75.6% had fever, 71.4% had erythema, and 46% had necrotic areas (Fig. 1).
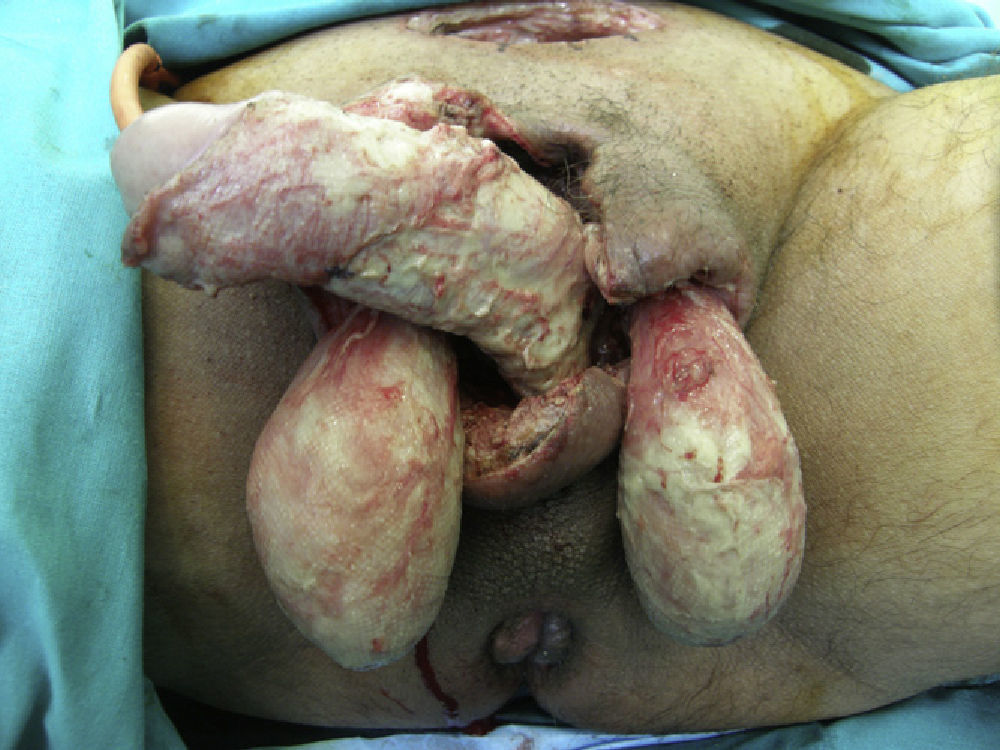
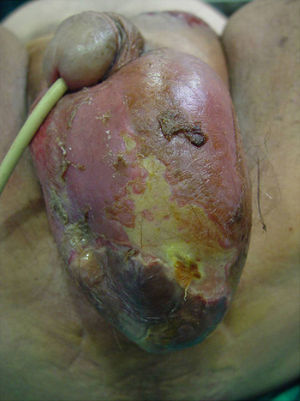
Once diagnosis had been confirmed by the presence of symptoms and the results of imaging tests, all patients required at least 1 surgical intervention, and 32.4% had to be admitted to the ICU because of severe sepsis or septic shock. The mean stay in the ICU was 7.83 (6.6) days (Fig. 2). Only chronic alcoholism had a statistically significant association with ICU stay (P<.001). The other underlying diseases (diabetes mellitus, ischemic heart disease, multiple conditions) were not associated with admission to the ICU.
Patients were transferred to the ward after leaving the ICU. The mean hospital stay was 27.54 (19.3) days. When diseases were classified independently, we observed that ischemic heart disease was significantly associated with a longer hospital stay (P=.007).
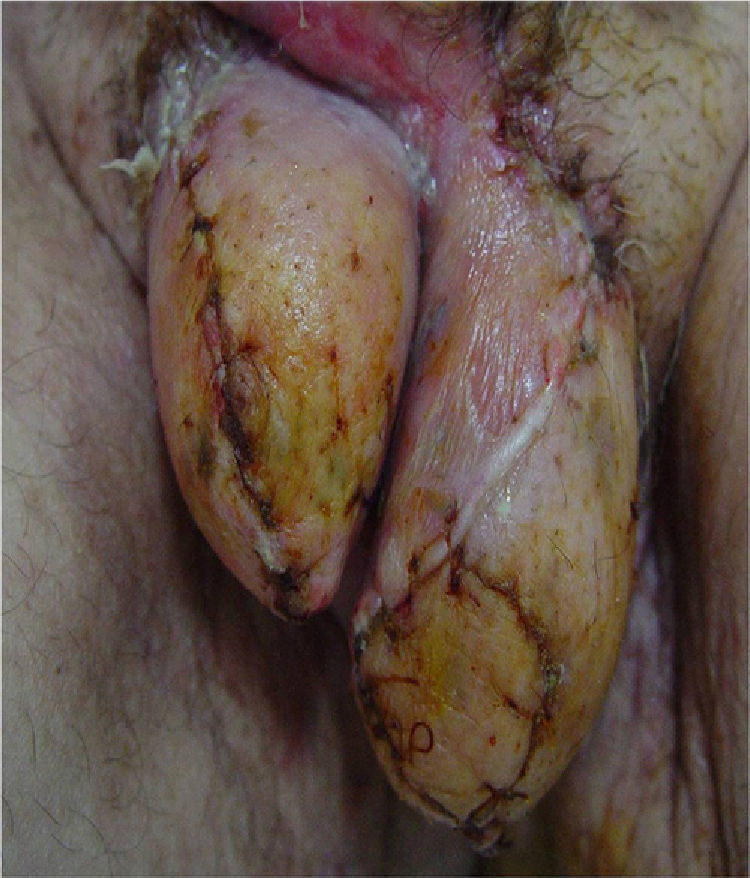
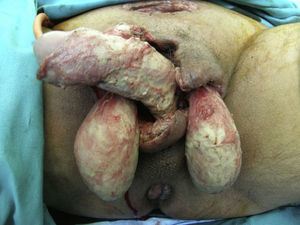
Once infection had been controlled and the surgical wound had healed, 32.4% of patients required surgical reconstruction. Secondary sutures were applied in 13.5% of cases, skin flaps in 5.4%, and grafts in 13.5% (Fig. 3).
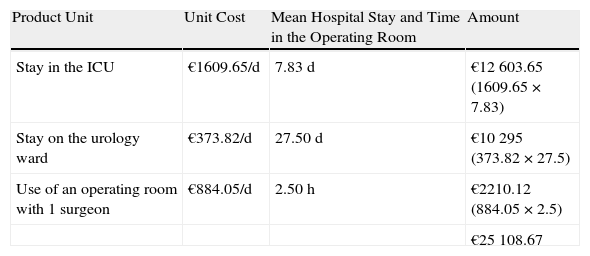
Based on available financial data and our results, we conclude that the mean overall health care cost of a patient with Fournier gangrene admitted to the ICU and requiring at least 1 surgical procedure, in addition to the procedure performed on admission (also included in the cost), is €25108.67 (Table 1).
Calculation of the Mean Overall Health Care Cost Generated by a Patient With Fournier Gangrene Admitted to the Intensive Care Unit (ICU) and Requiring 2 Procedures in the Operating Room.
| Product Unit | Unit Cost | Mean Hospital Stay and Time in the Operating Room | Amount |
| Stay in the ICU | €1609.65/d | 7.83d | €12603.65 (1609.65×7.83) |
| Stay on the urology ward | €373.82/d | 27.50d | €10295 (373.82×27.5) |
| Use of an operating room with 1 surgeon | €884.05/d | 2.50h | €2210.12 (884.05×2.5) |
| €25108.67 |
Infection was monomicrobial in 40.5% of patients and polymicrobial in 59.5%. The most commonly isolated microorganism was Escherichia coli (67.6%) followed by Bacteroides fragilis (21.4%). Other less commonly isolated agents were Enterococcus faecium (15.4%), Pseudomonas anaerobius (14.9%), Mycobacterium morganii (9.7%), Enterococcus faecalis (8.5%), and coagulase-negative staphylococci (6.7%).
The routine empirical antibiotic treatment administered in patients diagnosed up to 2006 was metronidazole (500mg/8h), together with cefotaxime (2g/d) and gentamicin adjusted for weight and renal function. From 2006 onwards, other regimens, such as meropenem 1g/8h, together with metronidazole 500mg/8h, were prescribed.
Only 5 patients (13.50%) died of the infection; differences in age between those who survived (69.6 years) and those who died (55.8 years) were statistically significant (P<.05). The origin of the infection was perianal abscess or fistula in 2 of the 5 patients who died; in the remaining 3 patients, no local primary cause was identified.
A significant association was detected between the presence of multiple conditions in 1 patient and mortality (P=.015). Similarly, when the diseases analyzed were classified separately, we only observed a significant association between ischemic heart disease and death due to Fournier gangrene (P=.002) (Table 2).
Association Between Study Variables and Admission to the Intensive Care Unit (ICU), Mean Hospital Stay, and Mortality.
| Study Variables | No. of Patients | Association With Stay in ICUa | Association With Mean Hospital Stay | Association With Mortality | |
| Yes | No | ||||
| Alcoholism | 8 | 29 | 8 (P=.000) | 29 (P=.760) | 29 (P=.283) |
| Diabetes mellitus | 16 | 21 | 4 (P=.399) | 24 (P=.357) | 2 (P=.875) |
| Ischemic heart disease | 9 | 28 | 4 (P=.376) | 12 (P=.007) | 4 (P=.002) |
| Multiple conditions | 12 | 25 | 6 (P=.114) | 23 (P=.002) | 4 (P=.015) |
| Perianal fistula/abscess | 11 | 26 | 4 (P=.740) | 23 (P=.444) | 2 (P=.589) |
| Urethral stricture | 6 | 31 | 3 (P=.315) | 14 (P=.791) | 0 (P=.290) |
| Previous catheter | 0 | 37 | – | – | – |
| Subsequent catheter | 23 | 14 | 6 (P=.311) | 25 (P=.395) | – |
| Admission to ICU | 12 | 25 | – | 27 (P=.993) | 3 (P=.157) |
| Monomicrobial infection | 15 | 22 | 4 (P=.536) | 29 (P=.698) | 2 (P=.959) |
| Polymicrobial infection | 22 | 15 | 8 (P=.003) | 18 (P=.698) | 3 (P=.678) |
| Secondary suture | 5 | 32 | 13 (P=.049) | 46 (P=.000) | – |
| Reconstruction with skin flap | 2 | 35 | 5 (P=.855) | 34 (P=.002) | – |
| Reconstruction with skin graft | 5 | 32 | 18 (P=.008) | 58 (P=.000) | – |
| Death | 5 | 32 | 3 (P=.157) | 7 (P=.009) | – |
When Fournier gangrene was first described, it was thought to affect men only. However, today we know that it can occur in up to 10% of women. In a series of 39 women diagnosed with Fournier gangrene, Sorensen et al.7 observed that mean age, race, prevalence of comorbid conditions, and number of debridements were similar to those of men. However, twice as many women required mechanical ventilation and dialysis, and hospital stay was longer and mortality greater than in men, although none of these findings was statistically significant. We recorded no cases in women or children in our series.
Common predisposing factors for Fournier gangrene include chronic alcoholism, systemic disorders, diabetes mellitus, chronic renal insufficiency, malignant neoplasm, and human immunodeficiency virus infection5,14; some series have reported an association between predisposing factors and mortality.1,15,16 These conditions are associated with reduced cell-mediated immune response, which favors infection. Most authors consider diabetes mellitus to be a risk factor for Fournier gangrene, although there is no agreement on whether it is associated with greater mortality.1 Erol et al.14 and Yanar et al.17 found that diabetes did not affect clinical outcome. In our study, 43.2% of patients had diabetes. We observed a statistically significant association between multiple conditions and mortality; however, of all the conditions analyzed only the association between ischemic heart disease and mortality was statistically significant. Ischemic heart disease was also associated with longer hospital stay.
There is no consensus on the variables that predict poor outcome in patients with Fournier gangrene. Some studies show that early and extensive debridement can significantly reduce mortality and that involvement of large areas is associated with greater mortality, since more interventions are necessary.9,10 In contrast, other studies suggest that the degree of involvement and the number of debridements are not predictors of outcome.5
Laor et al.18 developed the Fournier Gangrene Severity Index to determine the degree of severity and prognosis of the disease according to parameters such as temperature, heart rate, respiratory rate, hematocrit, leukocyte count, and serum levels of sodium, potassium, bicarbonate, and creatinine. The authors reported that a score of over 9 was associated with a 75% probability of death, whereas a score of 9 or less was associated with a probability of survival of 78%; both cutoff values have been validated elsewhere.9,19
It is important to recognize Fournier gangrene in the early stages, when cutaneous manifestations are minimal. However, diagnosis is difficult. Consequently, the condition is not recognized until an advanced stage (necrosis can spread at up to 2–3cm/h) and other types of necrotizing fasciitis may be classed as Fournier gangrene, despite affecting areas other than the perineum.4 Initial diagnosis is basically clinical, although, as with any type of necrotizing fasciitis, the histopathologic characteristics of Fournier gangrene (e.g., necrosis and suppuration of subcutaneous tissue, arteries, veins, superficial and deep fascia, and muscle) can also be determined.4
The first 24–48hours are characterized by nonspecific symptoms associated with hardening of the perineal area, mild fever, and erythema of the affected tissue. If the condition is not diagnosed in the early stages and the process follows its normal course, hemorrhagic blisters appear and can quickly become necrotic. Given the anatomical continuity between the fasciae, necrosis can spread to distant sites. Furthermore, the patient's general condition worsens, with progression to septic shock in almost 50% of cases.20
The results of imaging tests are sometimes useful for confirming the clinical suspicion, determining the extension of the disease, and evaluating the response to treatment.21 Radiography is more sensitive than physical examination for detection of subcutaneous emphysema in up to 89% of patients and reveals the pattern sometimes referred to as honeycomb scrotum.22,23 Some authors consider ultrasound to be the imaging technique of choice when diagnosis cannot be confirmed by clinical history and physical examination.24 Ultrasound makes it possible to identify gas in soft tissue, assess vascular flow in the testicles, and highlight subcutaneous edema and fluid collections.21 Furthermore, it can be performed at the bedside. This is an important advantage, given that some patients are hospitalized in the ICU or may be hemodynamically unstable, thus making it difficult to move them to the radiology department for a computed tomography (CT) scan. CT imaging does not usually show scrotal structures as well as ultrasound and often requires injection of contrast medium, which may not always be possible, as patients with Fournier gangrene often have renal failure.23,25 Nonetheless, some publications consider CT to be the procedure of choice since it enables evaluation of the extent of subcutaneous emphysema and fluid collections in soft tissue.21
Fournier gangrene is usually considered a polymicrobial infection, although not all the microorganisms involved are necessarily detected in culture. Both aerobes and anaerobes are almost always present, although anaerobes are isolated less frequently.3 The most commonly isolated species are E. coli, followed by streptococci, staphylococci, Pseudomonas aeruginosa, Bacteroides species, and clostridia. These entities are present in normal gastrointestinal and perineal flora.3,18,25 We isolated E. coli in 67.6% of patients, followed by B. fragilis in 21.4%.
The largest series to date (1726 cases) was published by Eke,11 who reported that the likely source of infection was the skin in 24% of cases, colon and rectum in 21%, and urinary tract in 19%. The source was unknown in 36% of patients.
The mortality rate of Fournier gangrene can be as high as 67%,3 despite new techniques applied in the ICU, extensive debridement, wound care, and broad-spectrum antibiotics. According to the literature, treatment is based on early and extensive debridement to remove infected and necrotic tissue, hemodynamic stabilization, and broad-spectrum antibiotics.4,26 The antibiotic regimen varies depending on the center and resistance to specific antibiotics in the geographic area where the microorganisms are isolated. Recent studies recommend starting empirical therapy with third-generation cephalosporins for gram-negative agents and metronidazole for anaerobes, with the possibility of adding aminoglycosides.1,4,21 An equally effective and easier alternative is monotherapy with broad-spectrum β-lactams or carbapenems of the ureidopenicillin family (piperacillin-tazobactam).1 At our center, we initially used 3 antibiotics: a third-generation cephalosporin, metronidazole, and, renal function permitting, gentamicin. Antibiotic resistance and the advent of agents with a broader spectrum, which are also easier to use, have led us to prescribe empirical carbapenems in monotherapy or combined with metronidazole, as this approach is effective in most patients.
Reconstructive procedures were necessary in 32.5% of the patients in our series. Of those who required a flap, 5.4% received vascularized musculocutaneous or fascial pedicle flaps. When it was necessary to use grafts, we chose thick grafts owing to their reduced contractility.
Fournier gangrene is a urological emergency with high mortality (20%–30%) despite early and appropriate treatment. There is no consensus on predictors of the disease. In most cases, the anorectal or genitourinary area is affected. When involvement of these sites occurs with underlying systemic diseases, such as diabetes mellitus and chronic alcoholism, susceptibility to polymicrobial infection increases. Although its incidence is low, Fournier gangrene generates high health care costs; therefore, primary and secondary preventive measures should be applied to correct the risk factors associated with the primary infection.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Jiménez-Pacheco A, et al. Análisis descriptivo y coste económico-sanitario de nuestra serie de 37 casos. Actas Dermosifiliogr. 2012;103:29–35.