Extramammary Paget disease (EMPD) has seldom been studied in Mediterranean populations. We aimed to review the characteristics of our patients with EMPD, the presence of a neoplasm in continuity, and the long-term course of the disease.
Patients and methodsRetrospective observational study of 27 patients diagnosed with EMPD between 1990 and 2015. All clinical and pathology findings related to clinical course and outcomes were retrieved for analysis.
ResultsTwenty patients were women and 7 were men. Ages ranged from 42 to 88 years (median, 76 years). Lesions were in the following locations: vulva (16 cases), pubis–groin (5), perianal region (4), and axilla (2). Time from onset to diagnosis ranged from 1 to 60 months (median, 12 months) and maximum lesion diameter from 20 to 140mm (median, 55mm). In 3 cases (11.1%) EMPD was a secondary condition. None of the lesions developed on a previous cutaneous adnexal adenocarcinoma. Ten of the 24 primary EMPDs (41.7%) invaded the dermis. Eight of the 27 patients (29.6%) experienced local recurrence after the initial surgical treatment. Three patients (11.1%) died as a consequence of metastasis from the EMPD.
ConclusionsThe presence of an underlying cutaneous adnexal adenocarcinoma is uncommon, but it is not unusual to find an extracutaneous adenocarcinoma in continuity. Although EMPD is a slow-growing tumor, dermal invasion is frequent and metastasis is not uncommon. Local recurrence is common even after excision with wide margins and may be delated, so long term follow-up is essential.
Existen pocos estudios sobre la enfermedad de Paget extramamaria (EPEM) en la población mediterránea. Nuestro objetivo fue revisar las características de nuestros pacientes con EPEM, su asociación con neoplasia en continuidad y su evolución a largo plazo.
Pacientes y métodosRealizamos un estudio observacional retrospectivo sobre 27 pacientes diagnosticados de EPEM entre 1990-2015. Las historias clínicas fueron revisadas retrospectivamente para obtener los datos clínico-patológicos y de seguimiento.
ResultadosSe trata de 20 mujeres y 7 varones de entre 42 y 88 años de edad (mediana de 76 años). Las lesiones se localizaron en la vulva (16 casos), en el pubis-región inguinal (5), en la región perianal (4) y en la axila (2). El tiempo de evolución al diagnóstico osciló entre 1 y 60 meses (mediana de 12 meses) y el diámetro máximo entre 20 y 140mm (mediana de 55mm). En 3 casos (11,1%) la EPEM fue secundaria. Ningún caso se desarrolló sobre adenocarcinoma anexial cutáneo previo. Diez de 24 EPEM primarias (41,7%) presentaban invasión de la dermis. Ocho de los 27 pacientes (29,6%) presentaron recidiva local tras el tratamiento quirúrgico inicial. Tres pacientes (11,1%) fallecieron a consecuencia de metástasis de la EPEM.
ConclusionesLa presencia de un adenocarcinoma anexial cutáneo subyacente es poco frecuente pero no es rara la existencia de un adenocarcinoma extracutáneo en continuidad. A pesar de que la EPEM suele evolucionar lentamente, es frecuente la invasión de la dermis y no son excepcionales las metástasis. Las recidivas locales son frecuentes a pesar de la extirpación con márgenes amplios y pueden ser tardías, por lo que es preciso un seguimiento a largo plazo.
Extramammary Paget's disease (EMPD) is an uncommon disease caused by the intraepidermal proliferation of malignant cells of glandular origin outside the areola complex.1,2 Despite its low incidence, several large studies have recently been published in Europe, Asia, and the United States.3–8 Most of these, however, are epidemiological studies and their findings show considerable differences in terms of clinical characteristics and a possible link to underlying malignancy. Some of these differences could be due to differences in racial backgrounds, while others could be due to differences in design. In view of these discrepancies and the scarcity of studies on EMPD in southern Europe, we decided to analyze patients diagnosed with EMPD at our hospital to provide an overview of clinical characteristics, association with underlying malignancy contiguous with the affected skin, and long-term outcomes.
Patients and MethodsWe conducted a retrospective, observational study of 27 patients diagnosed with EMPD between 1990 and 2015. All cases registered as EMPD in the pathology laboratory's database were analyzed. The diagnostic criteria were presence of an intraepidermal proliferation of predominantly isolated or small groups of atypical cells with a pagetoid appearance in the parabasal and spinous layers. All tumors stained positively for keratins in the immunohistochemical study (in particular, cytokeratin 7, epithelial membrane antigen, and GCDFP-15). Negative staining for S100, Melan-A, and HMB-45 ruled out a melanocytic tumor in all cases. Cases with underlying adenocarcinoma were diagnosed as secondary EMPD. Patients were monitored clinically by the dermatology, gynecology, and plastic surgery departments. Their medical charts were retrospectively reviewed to collect data on race, sex, age at diagnosis, date of diagnosis, time to diagnosis, tentative clinical diagnosis prior to biopsy, lesion location and diameter, treatment, number of local recurrences, presence of invasive disease, presence of underlying adnexal adenocarcinoma and/or extracutaneous carcinoma in continuity, development of metastasis, date of last follow-up visit, and date of death and cause (EMPD vs. other causes).
Data were analyzed using the SPSS statistical package, version 17.0 for Windows. Categorical variables were compared using the Fisher exact test, and continuous variables were compared using the t test for normally distributed data and the Mann-Whitney U test otherwise. Statistical significance was set at P<.05. Study variables were compared according to patient sex and the presence or absence of dermal invasion and local recurrence. Patients with primary and secondary EMPD and those who died of EMPD and those who did not were also compared.
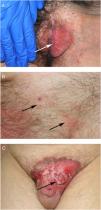
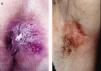
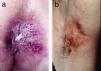
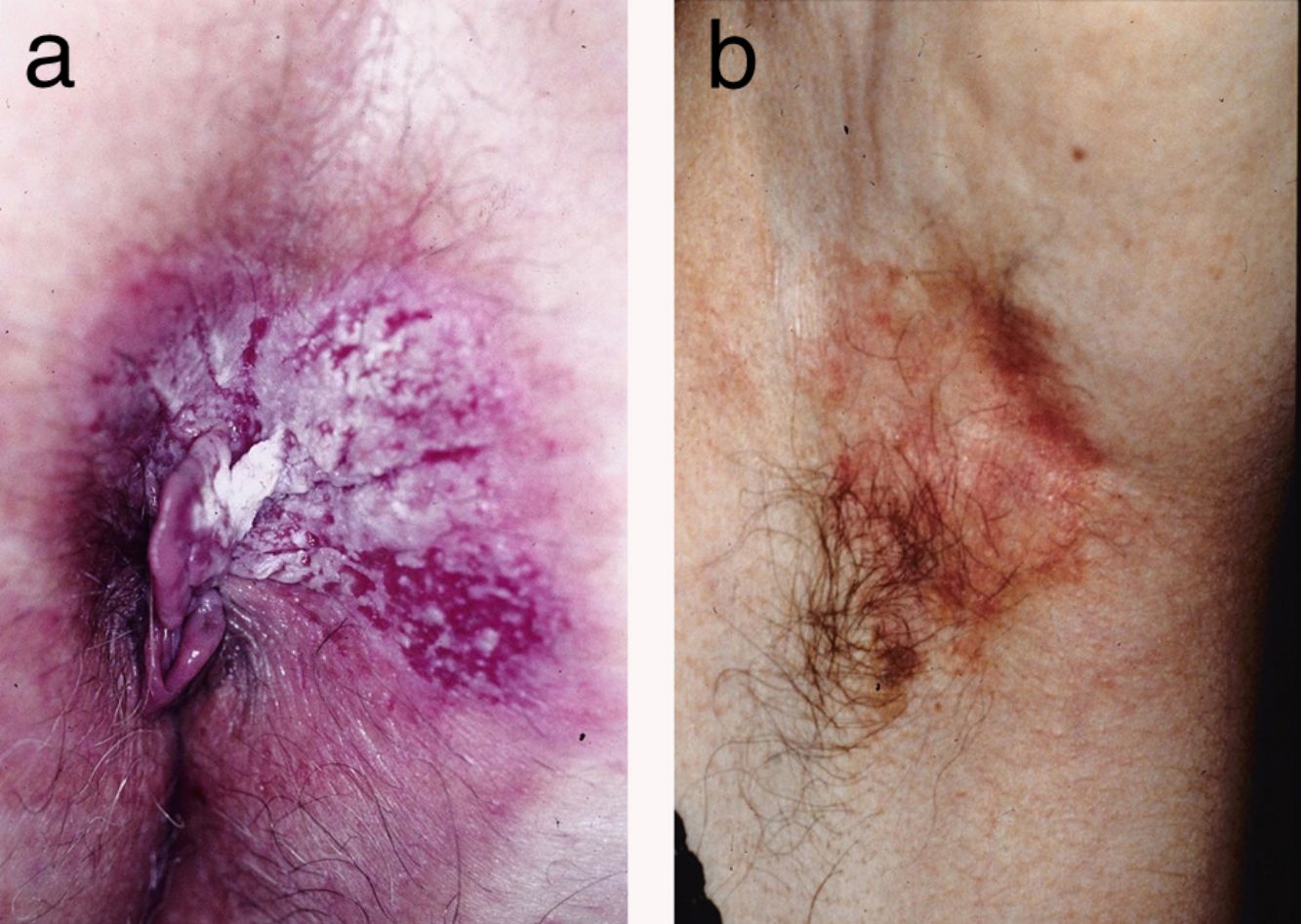
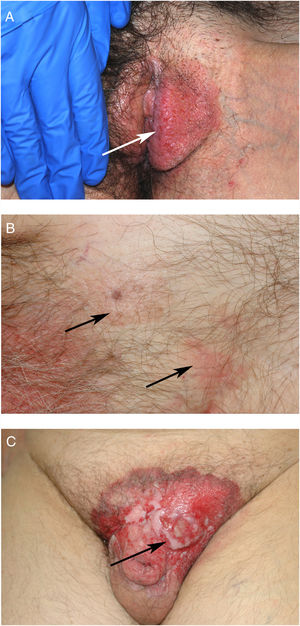
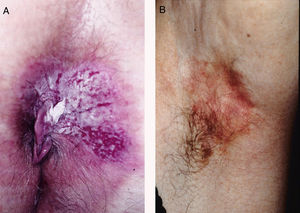
ResultsWe studied 20 women and 7 men, all white. Median age at diagnosis was 76 years (range, 42–86 years). Most patients presented noninfiltrated, slightly erythematous plaques; some of the lesions had well-defined margins but others had poorly defined margins (Figs. 1 and 2). Tentative clinical diagnoses before biopsy (n=26) were recorded for 21 patients. The most common diagnoses were lichen simplex (5 cases), eczema (3 cases), leukoplakia-erythroplakia (3 cases), and squamous cell carcinoma (2 cases). Additional diagnoses (1 case each) were Bowen disease, superficial basal cell carcinoma, intertrigo, condyloma, bowenoid papulosis, dermatophytosis, psoriasis, atrophy, and ulcer. EMPD was only suspected in 4 cases. Time to diagnosis and lesion location are detailed in Table 1. Maximum lesion diameter ranged from 20mm to 140mm (median, 55mm). There were 3 cases of secondary EMPD: 2 rectal adenocarcinomas and 1 breast tumor in the left axilla. None of EMPDs was associated with an underlying apocrine adenocarcinoma. Ten (41.7%) of the 24 patients with primary EMPD had dermal invasion. Depth of invasion was less than 1mm in 9 patients and 6cm in 1 patient who had initially refused treatment (Fig. 1). Surgical excision was performed in 25 patients (22 of the 24 patients with primary EMPD and the 3 patients with secondary EMPD). One patient refused surgical treatment, opting for carbon dioxide laser treatment instead, and another died of a cause other than EMPD before treatment was possible.
Clinical Characteristics of Patients With EMPD Stratified by Sex.a
| Female (n=20, 74.1%) | Male (n=7, 25.9%) | ||
|---|---|---|---|
| Age at diagnosis, y | |||
| Median (range), 76 (42–86) | 73.5 (42-86) | 79 (55-82) | P=.183a |
| Location | |||
| Vulva, 16 (59.3%) | 16/20 (80%) | 0/7 (0%) | |
| Pubis-groin, 5 (18.5%) | 0/20 (0%) | 5/7 (71.4%) | |
| Perianal, 4 (14.8%) | 2/20 (10%) | 2/7 (28.6%) | |
| Axilla, 2 (7.4%) | 2/20 (10%) | 0/7 (0%) | |
| Diameter, mm | |||
| Median (range), 55 (20-140) | 57.5 (20-140) | 37 (25-105) | P=.543c |
| Time to diagnosis, mo | |||
| Median (range), 12 (12–60) | 12 (3-36) | 6 (1-60) | P=.326a |
| Secondary EMPD, 3/27 (11.1%) | 1/20 (5%) | 2/7 (28.6%) | P=.156 |
| Invasive primary EMPD, 10/24 (41.7%) | 7/19 (36.8%) | 3/5 (60%) | P=.615 |
| Treatment | |||
| Surgery, 25 (92.6%) | 18/20 (90%) | 7/7 (100%) | |
| Radiotherapy, 3 (11.1%) | 2/20 (10%) | ||
| Imiquimod, 7 (25.9%) | 4/20 (20%) | 3/7 (50%) | |
| CO2 laser, 1 (3.7%) | 1/20 (5%) | 0/7 (0%) | |
| Local recurrence, 8/27 (29.6%) | 7/20 (35%) | 1/7 (14.3%) | P=.628 |
| Metastasis, 3/27 (11.1%) | 2/20 (10%) | 1/7 (14.3%) | P>.99 |
| Death due to EMPD, 3/27 (11.1%) | 2/20 (10%) | 1/7 (14.3%) | P>.99 |
Abbreviations: CO2, carbon dioxide; EMPD, extramammary Paget disease.
Eight of the 27 patients (29.6%) experienced at least 1 local recurrence after primary surgery. They all had primary EMPD and 3 had clear surgical margins. Four of the patients had multiple local recurrences: one patient had 6 recurrences, another had 5 recurrences, and two had 3 recurrences. Time to recurrence was less than 3 years after primary surgery in all 8 patients, but 1 of these developed successive recurrences over a period of 13 years. Follow-up time ranged from 1 month to 276 months (median, 60 months). Three patients (11.1%) developed distant metastasis and died as a result.
The clinical characteristics of the patients stratified by sex are shown in Table 1. Compared with primary EMPD, secondary EMPD was associated with a younger median age at diagnosis (69 vs. 77 years) and a smaller median diameter (30mm vs. 57.5mm). Patients who developed local recurrence had a larger median diameter than those who did not (60mm vs. 45mm) and they were also younger at diagnosis (median age, 65 vs. 77.5 years). Patients who died due to EMPD also had a larger median diameter than those who did not (80mm vs. 52.5mm). None of above differences, however, were statistically significant. Differences in age, lesion diameter, and time to diagnosis between patients with invasive and noninvasive EMPD were also nonsignificant. Invasive disease, however, was significantly associated with a risk of metastasis and death due to EMPD (the 3 patients who died had invasive disease, P=.041).
DiscussionExtramammary and mammary Paget disease are both considered to be intraepidermal adenocarcinomas.1 Mammary Paget disease is caused by the spread of an underlying carcinoma (mainly ductal) to the epidermis of the areola complex and is usually detected by histology. EMPD is a more heterogeneous entity. Because of its histologic similarity to mammary Paget disease and its predilection for areas rich in apocrine glands, it has traditionally been considered to originate from apocrine adenocarcinoma.1 There are, however, many cases in which an underlying apocrine adenocarcinoma is not detected. Some authors have argued that this may be because in situ disease of apocrine glands is overlooked in the histologic examination.1 EMPD can also be caused by intraepidermal spread from a visceral adenocarcinoma in continuity with the skin, such as an uterine, urothelial, or anal adenocarcinoma.2 EMPDs without an underlying adenocarcinoma are classified as primary, while those with an underlying adenocarcinoma are classified as secondary.
The most widely accepted theory at present is that most EMPDs develop as intraepithelial tumors and are therefore primary. They are believed to originate from apocrine or eccrine cells in the epidermis. Another hypothesis is that they arise from pluripotent epidermal stem cells such as the clear pagetoid cells of the nipple described by Toker, which have been identified in perianal skin.9 As mentioned, secondary EMPD can be caused by an underlying apocrine adenocarcinoma or by intraepithelial spread from a contiguous visceral adenocarcinoma. Reports on the frequency of apocrine adenocarcinomas invading the epidermis are inconsistent. In one large review of the literature, 46 of 153 patients had an underlying cutaneous adnexal adenocarcinoma; this corresponds to a rate of approximately 30%.10 Other studies, by contrast, have detected very few cases. In one series of 38 patients with penoscrotal EMPD, just 3 patients had an underlying adnexal adenocarcinoma.11 We did not observe any cases of EPDM arising in an apocrine adenocarcinoma in our series, leading us to believe that, at least in our population, this type of EPDM is rare. The opposite situation would appear to be much more common, that is EPDM arising in the epidermis and then progressing to dermally invasive adenocarcinoma. Conflicting rates have also been reported for the prevalence of EMPD associated with a visceral adenocarcinoma in continuity. While some studies have reported very low or inexistent rates (e.g., 0 cases in 28 patients with penoscrotal EPDM11), a large recent study detected contiguous extracutaneous adenocarcinoma in 37 of 161 patients (23%), and reported a higher prevalence in patients with EPMD of anal-rectal, genital, or urothelial origin.7 The association appears to be even higher for perianal EMPD, with some literature reviews reporting underlying visceral adenocarcinoma in approximately 80% of cases, although the association may be overestimated as the reviews were of published cases.12,13 In our series, there were 3 cases of secondary EMPD (2 associated with rectal adenocarcinoma and 1 with breast carcinoma in the axilla and EMPD on the overlying skin). This corresponds to a prevalence of 11.1% overall and 50% of all perianal cases.
The potential association between EMPD and prostate adenocarcinoma is also noteworthy. Elevated prostate-specific antigen (PSA) levels and immunohistochemical expression of PSA in some EMPDs suggest that this tumor may arise from prostate adenocarcinoma. However, in a recent study of EMPD, high PSA levels and positive immunohistochemical staining for PSA were observed in some patients without prostate adenocarcinoma,14 indicating that the association may be overestimated because of the advanced age of patients with EMPD. None of the patients in our series had prostate adenocarcinoma.
The possible link between EMPD and distant visceral malignancy is also controversial,8,10 and no clear pathogenic relationship has been established. Although dermal metastases from visceral adenocarcinoma can invade the epidermis following a pagetoid pattern,15 they cannot strictly be considered to be EPDMs but rather distant cutaneous metastases with an epidermotropic component. They can usually be distinguished from true EMPDs as the dermal component is larger than the epidermal component. In a recent study, Schmitt et al.7 were unable to confirm whether the coexistence of EMPD and underlying malignancy represented a true association or was a coincidental finding related to age. The authors suggested that cancers that are not in continuity with the skin should not be included under the umbrella of EMPD-associated cancers.
Considerable differences in EMPD incidence, sex, and lesion location have been observed between patients of different racial backgrounds. The estimated annual incidence of EMPD is 0.9 cases per million inhabitants in the western world and 10 cases per million inhabitants in Asia.5,16 If we extrapolate the data from our series, the annual incidence in our population would be approximately 1 case per million inhabitants, which is similar to rates reported by other studies of western populations. Twenty (74.01%) of the 27 patients in our series were women. This female predominance has been reported in other studies of white patients,7 and contrasts with reports from Asia, where EMPD appears to be more common in males.6 The most common sites involved in our series were the vulva followed by the perianal area in women and the pubic-inguinal area followed by the perianal area in men. Again, these findings are consistent with reports from other studies of white patients.7 There were no cases affecting the penis in our series and just 1 patient had scrotal involvement extending from the pubis. Data regarding the age of patients with EMPD are more consistent in the literature. Coinciding with most other reports, the median age of patients in our series at diagnosis was above 70 years.7
EMPD is generally described as a slow-growing, erythematous, round, slightly raised plaque with a typically well-demarcated margin and on occasions a scaly surface.17 Mucosal involvement can present as leukokeratosis. The presenting symptom, particularly in lesions involving the vulva, is pruritus and EMPD must be suspected in patients with very persistent vulvar itching.17,18 In general, however, EMPD, is not suspected before biopsy. In our series, a tentative diagnosis of EMPD was only considered in 4 patients. The low clinical specificity of EMPD lesions would explain why the mean time to diagnosis described in the literature is approximately 2 years.18,19 In our series, a diagnosis was made after a median period of 12 months.
Even when EMPD does not arise in an underlying adenocarcinoma, if allowed to progress, it can invade the dermis and cause regional lymph node and even distant metastasis.6 Invasive primary EMPD was reported in between 15% and 40% of cases described in a recent review.2 In our study, the rate was 41.67% (10 of 24 cases), which is similar to previous reports for Spain.20 Signs of poor prognosis in invasive EMPD are a greater depth of invasion, elevated serum carcinoembryonic antigen levels, lymphovascular invasion, and number of affected regional lymph nodes.6,19,21 EMPDs with an invasion depth of less than 1mm are considered to be minimally invasive,18 while those extending deeper than 4mm are associated with an increased risk of metastasis.6 In one study, 114 (38%) of 301 patients with invasive EMPD developed metastasis (regional lymph node involvement in 21% of cases and distant metastasis in 17%).6 In our series, 3 of the patients with primary invasive EMPD developed distant metastasis; this corresponds to 12.5% of all primary EMPDs and 30% of all invasive primary EMPDs. All the patients died as a result. Coinciding with previous reports,2 none of the patients with noninvasive EMPD in our series developed metastasis.
Surgical excision is the treatment of choice for EMPD.2 Some authors recommend excision with a 1-cm margin for well-defined lesions and a 2-cm margin otherwise.2,19,22 Preoperative biopsy mapping can be useful for poorly defined EMPDs and when margins of 2cm cannot be achieved.22 Recurrence rates after conventional surgery are, however, quite high and range from 12% to 58%, with a mean of 33%.18 In our series, 8 patients (29.6%) developed local recurrence and several patients developed multiple recurrences despite preoperative biopsy mapping of ill-defined tumors. Mohs micrographic surgery (MMS) can reduce recurrence rates to between 8% and 16%.23,24 One group of authors recently suggested that immunohistochemical staining for cytokeratin 7 during MMS could further reduce the risk of recurrence, but this process requires experience and can lengthen operating time.25 One technique that has been proposed for shortening operating times compared with MMS is frozen section–guided wide local excision.11 Another issue to bear in mind is that clear margins are not a guarantee of cure, as high recurrence rates have been observed with both diseased margins (70%) and clear margins (37.5%).26 These findings suggest that mutilating surgery to achieve margin clearance is not always justified. Complex cases should be discussed in a multidisciplinary team and decisions tailored to patient and family preferences and the patient's clinical condition. In one study, topical imiquimod used to treat EMPDs that could not be surgically excised resulted in complete response rates ranging from 56% to 75%, although over half of the patients developed local recurrence.2 Radiotherapy can also be used as an adjunct to surgery or as an alternative in patients in whom surgery is contraindicated or unfeasible.27 Photodynamic therapy has produced similar response rates to imiquimod.28 As regional lymph node metastasis occurs in 8.5% to 26% of cases,29 some studies have evaluated the usefulness of sentinel lymph node (SLN) biopsy,30,31 but its prognostic value is controversial and its therapeutic value has yet to be determined. Lymph node dissection tends to be performed in patients with a positive SLN biopsy or clinical evidence of regional lymph node involvement.2 However, identification of the SLN can be difficult as EMPDs can be extensive and are usually located close to the inguinal lymph nodes. Accordingly, and in line with guidelines for vulvar cancer, some teams opt for direct lymph node dissection rather than SLN biopsy in patients with invasive EMPD extending deeper than 1mm in the vulvar or perianal region.2 There are no standardized treatments for distant metastases of EMPD, but most studies published to date have used different combinations of carboplatin or cisplatin, taxanes, and/or 5-fluorouracil.2,32
Some authors recommend intensive staging tests (e.g., Papanicolaou smear screening, mammography , PSA measurement, colonoscopy, and urine cytology) to rule out associated tumors, in addition to age-appropriate screening.7 Others, however, have recently questioned the usefulness of colonoscopy and recommend ruling out additional tumors only when the EMPD is in continuity with the anus, urethra, or vagina.2,33 Accordingly, urine cytology and Papanicolaou smear screening would only be indicated for genital EMPD, whereas rectoscopy would be indicated for perianal EMPD. Long-term clinical follow-up is particularly important because patients with clear surgical margins can develop disease recurrence,26 as observed in 3 of our patients. Despite the lack of large series and considering the risk of metastasis in patients with invasive EMPD, imaging tests may be indicated in the first years of follow-up.
The limitations of the present study are its retrospective observational design and small sample.
In this Mediterranean series, EMPD was more common in women, in the vulva, and in people over 70 years of age. Underlying apocrine adenocarcinoma is very rare, but invasive disease was detected at diagnosis in 41.7% of patients. Secondary EMPD accounted for 11.1% of all cases and 50% of perianal cases. Local recurrence was common, particularly in patients with lesions with a large diameter, and some patients experienced multiple recurrences. Although EMPD progresses slowly, 3 of the patients in our series died of metastases from EMPD (11.1% of all patients and 30% of patients with invasive primary EMPD). Considering that local recurrence is common, irrespective of surgical margins, and may occur after more than 10 years of treatment, long-term follow-up of EMPD patients is required.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Marcoval J, Penín RM, Vidal A, Bermejo J. Enfermedad de Paget extramamaria. Actas Dermosifiliogr. 2020;111:306–312.