Confocal microscopy (CM) is an imaging technique that permits real-time visualization of skin structures with a resolution approaching that of conventional histology.1 This technique has been applied in several skin diseases, in particular, tumors such as basal cell carcinoma (BCC) or squamous cell carcinoma. With this technique, the skin can be imaged directly (in vivo) or surgically removed pieces can be examined (ex vivo). In the latter case, the use of fluorophores has improved the quality of the images obtained with a technique known as fluorescence CM (FCM).
Several different dyes have been used in FCM, such as methylene blue or toluidine blue; however, acridine orange (AO) is the most widely used.2 AO binds specifically to DNA or RNA of living cells, acting as a fluorophore that is excited at a specific wavelength and then then emits fluorescence. Nuclear cells can therefore be marked, and they are observed as brilliant white structures in FCM mosaics.3 Structures that do not have a nucleus, such as collagen bundles that form part of the dermis, would emit weak fluorescence or not at all. AO can therefore increase image contrast 100-fold.4 It is also important to point out that AO does not lead to sample degeneration and subsequent staining with traditional stains such as hematoxylin-eosin is still possible.5
To date, in images of FCM published in the scientific literature, fluorescence emitted by AO has been translated by microscopy to a final greyscale or dichromatic image.6 Despite the excellent contrast between structures and the strong histopathologic correlation shown by these images, the final greyscale mosaics are difficult to interpret for dermatologists and pathologists who are not expert in CM.
Recently, our group has described a new technique for obtaining FCM images using a 3 color scale (FCM-3CS), through the joint use of AO and ethidium bromide (EB).7
In this technique, the resected pieces are immersed in liquid nitrogen, thereby ensuring an almost instantaneous freezing. They are then sectioned in a cryostat in rapid cuts measuring 20–30 μm thick. After sectioning, the sample is stained by pouring on a mixture of AO 0.1 mM and EB 0.25 nM, and leaving the solution to act for a minute. After this brief processing, the sample is placed under a commercially available confocal microscope, Nikon A1R+ (Nikon Corporation®, Japan). Once under the microscope, the sample is excited simultaneously with 2 different wavelengths, 405 nm and 488 nm. The microscope collects fluorescence emitted by the sample after excitation to obtain images with a 3-color scale. The process requires between 10 and 15 minutes to obtain the final color mosaics.
These color images are the result of the joint action of the 2 fluorophores applied to the sample. EB is a fluorophore that binds specifically to DNA in cells that have lost membrane integrity.8 EB passes through the damaged nuclear membranes after freezing, binding with high affinity to DNA and thus staining these cell nuclei. In this way, when the sample is excited with laser light at 405 nm, EB emits red fluorescence, marking the nuclei with high precision. The dermis and anucleate structures emit green fluorescence from AO after excitation at 488 nm. The weak blue fluorescence originates from intrinsic fluorescence of the tissues. All this fluorescence is detected by the microscope, and as a result, the final images are obtained on a 3-color scale.
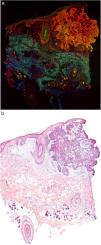
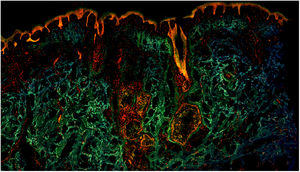
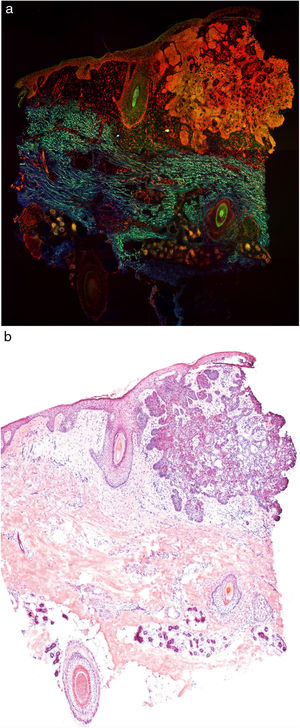
Fig. 1 shows how a fragment of healthy skins looks with this technique. Nucleated structures emit red fluorescence, which contrasts significantly with green fluorescence, yielding images that are very intuitive and easy to interpret, with a very high resolution. This resolution enables the skin tumor margins to be delimited with high precision, for example in the case of BCC shown in Fig. 2A. Note the complete correlation with classic hematoxylin-eosin staining (Fig. 2b). After processing, in relation to images H–E, we do not observe significant changes in image quality.
The main application of ex vivo CM currently is in oncological surgical processes, such as Mohs surgery. It can be expected that in coming years, the cost of devices needed for CM will come down, making it a cost-effective technique that could be incorporated progressively into everyday clinical practice.
In conclusion, images with color FCM images are significantly simpler to interpret than greyscale images for dermatologists and pathologists who are not expert in CM. Furthermore, thanks to processing by sample freezing, the sample is completely flat. Therefore, complete sample images can be obtained, without losing mosaics due to sample folding. These are all important advantages compared with traditional images obtained with CM. Nevertheless, more studies are needed to validate this new technique.
FundingThis study did not receive any funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sendín-Martín M, Domínguez-Cruz JJ, Levitsky K-L, Conejo-Mir Sánchez J. Microscopía confocal de fluorescencia ex vivo en escala de tres colores (mcf-3cs): una nueva técnica de imagen. Actas Dermosifiliogr. 2020. https://doi.org/10.1016/j.ad.2019.04.010