Erythrodermic psoriasis (EP) is a rare and severe form of the disease characterized by diffuse redness and scaling, covering more than 90% of the body surface area (BSA) and affects 1–2.25% of patients with psoriasis.1 Although a complex interplay of Th1, Th2, and Th17 inflammatory pathways seems to be involved in EP pathophysiology, recent findings showed that IL-17 responses are the dominant signal in erythrodermic psoriasis, suggesting that anti-IL-17 agents could represent an effective therapeutic option.2
Brodalumab is a fully human monoclonal antibody that targets the IL-17 receptor A, inhibiting the biological activity of different isoforms of IL-17 (IL-17A, A/F, F, C and E) with a different mechanism of action when compared to other anti-IL17A biologic drugs.3,4 A phase III study in Japan showed that brodalumab significantly improved the signs and symptoms of patients with EP throughout 52 weeks, with a favorable safety profile, suggesting that this drug might be a promising treatment option for EP.5 A small number of real-life cases of EP successfully treated with brodalumab have been described, although long-term efficacy and safety data is lacking.6,7 We report three cases of real-life patients with EP achieving complete clearance after brodalumab treatment, with no adverse events reported during a follow-up period of 52–116 weeks.
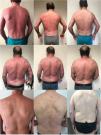
The first case is a 40-year-old male with a long history of plaque psoriasis, several comorbidities (dyslipidemia and excessive alcohol use) and previous multiple failures to topicals, acitretin, ciclosporin, infliximab and ustekinumab. The patient presented with severe EP, a BSA of 100%, a PASI score of 48 and a DLQI of 27 (Fig. 1a), without psoriatic arthritis (PsA). Given the high impact on quality of life and the intense itch, the patient initiated brodalumab at labeled dosage. Significant improvement was observed: 6 weeks after the first injection, PASI score decreased to 21 (ΔPASI 57), BSA involvement decreased to 70% and the itching also disappeared (Fig. 1b). After 14 weeks follow-up, BSA involvement was <1%, the absolute PASI (aPASI) was <1 (ΔPASI ∼100) with no impact on quality of life (DLQI 0) (Fig. 1c). No adverse events have been observed during treatment with brodalumab. After 104 weeks follow-up, the disease is still in remission.
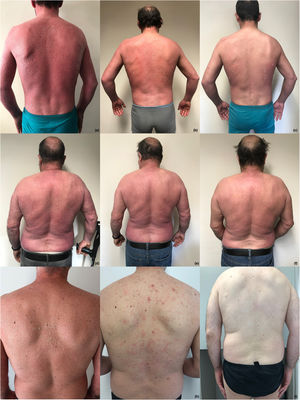
Case 1 – patient with erythrodermic psoriasis at baseline (a) and after 6 (b) and 14 (c) weeks of first Brodalumab injection; case 2 – patient with erythrodermic psoriasis at baseline (d) and after 4 (e) and 8 (f) weeks of first Brodalumab injection; case 3 – patient with erythrodermic psoriasis at baseline (g) and after 4 (h) and 8 (i) weeks of first Brodalumab injection.
The second case is a 59-year-old male with a long history of plaque psoriasis and multiple comorbidities (obesity, dyslipidemia, hypertension, and excessive alcohol use), previously treated with topicals, acitretin and phototherapy. The patient presented with a BSA involvement of 90%, a PASI score of 43 and a DLQI score of 25 (Fig. 1d), without PsA. The patient referred no articular pain and tested negative for latent tuberculosis (TB) infection. Brodalumab was initiated at labeled dose and significant improvement was observed just 4 weeks after the first injection: PASI score decreased to 7 (ΔPASI>75) and BSA involvement decreased to 10% (Fig. 1e). After 8 weeks follow-up, the BSA was <1%, aPASI<1 (ΔPASI ∼100), with no impact in quality of life (DLQI 0) (Fig. 1f) and no adverse events, which is maintained after 52 weeks of follow-up.
The third case is a 43-year-old male, smoker, with plaque psoriasis since he was 16-years old, referred due to a worsening of his psoriasis in the past 5-years. Patient presented a diffuse cutaneous redness with a BSA of 90%, a PASI score of 31 and a DLQI of 18 (Fig. 1g), without PsA, and was naïve to systemic therapy. As comorbidities, this patient had a confirmed hepatitis C (HCV) infection and tested positive for latent TB. After curing HCV (sofosbuvir/velpatasvir) and receiving treatment for latent TB (isoniazid), the patient was started on brodalumab at the labeled dose. Remarkable improvement was observed: PASI 90 response and DLQI 0 at week 4 (Fig. 1h) and PASI 100 response and DLQI 0 at week 8 (Fig. 1i). After 116 weeks follow-up, aPASI 0 and DLQI 0 are maintained, with no observed adverse events.
All cases showed a high efficacy and good safety profile of brodalumab in the treatment of EP in the real-world setting, despite failure or contra-indication to previous treatments. Brodalumab also showed a rapid onset of action, returning patients’ quality of life in the short and long-term. Although specifically targeting Th17 mediated inflammation, the clinical benefit of brodalumab in erythrodermic psoriasis might be explained by its unique mechanism of action, inhibiting a wider spectrum of the IL-17 cytokine family. In fact, the inability of other biologic agents (anti-IL17A, -IL12/23 and -IL23) to block the entire IL-17 cell line, in particular IL-17C and IL17-E, might explain the appearance of flares and secondary therapeutic failures as reported here in case 1 and in another previously published case.4,6,8,9
In conclusion, brodalumab represents a good option for EP as both rapid onset of action and favorable long-term efficacy/safety profile are needed. Further studies with larger sample sizes are needed to confirm brodalumab's clinical benefit for this challenging form of psoriasis.
Conflict of interestF. Mota has received honoraria for acting as a consultant and/or as a speaker for Galderma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer and Sanofi Genzyme.
He has also worked as a co-investigator in clinical trials supported by Amgen and Novartis.
P Mendes-Bastos has received honoraria for acting as a consultant and/or as a speaker for AbbVie, Janssen, Novartis, LEO Pharma, Almirall, Sanofi, Viatris, L’Oréal and Cantabria Labs. He has also worked as a principal investigator in clinical trials supported by AbbVie, Sanofi and Novartis.