Epidermal nevi are hamartomatous lesions derived from the epidermis and/or adnexal structures of the skin; they have traditionally been classified according to their morphology. New variants have been described in recent years and advances in genetics have contributed to better characterization of these lesions and an improved understanding of their relationship with certain extracutaneous manifestations. In the first part of this review article, we will look at nevi derived specifically from the epidermis and associated syndromes.
Los nevus epidérmicos son hamartomas originados en la epidermis y/o en las estructuras anexiales de la piel que se han clasificado clásicamente partiendo de la morfología. En los últimos años se han descrito variantes nuevas y se han producido avances en el campo de la genética que han permitido caracterizar mejor estas lesiones y comprender su relación con algunas de las manifestaciones extracutáneas a las que se han asociado. En esta primera parte revisaremos los nevus derivados de la epidermis y los síndromes que se han descrito asociados a ellos.
A hamartoma is a tumor-like malformation, usually congenital, arising due to an abnormal mixture in the distribution or proportions of mature, constitutive tissue elements. It is not a neoplasm because the tissues do not undergo autonomous growth. Skin hamartomas are called nevi. There is a certain degree of confusion regarding the definition of the term epidermal nevus. Most articles in the scientific literature consider epidermal nevi to refer to lesions derived from the epidermis or from adnexal epithelial cells. Some texts on skin pathology, however, define epidermal nevi as those lesions derived from epidermal keratinocytes (herein denoted keratinocytic nevi) excluding nevi derived from adnexal structures.1,2 In this article, we will refer to both nevi derived from the epidermis and those derived from adnexal structures. The review will be divided into 2 parts. Table 1 summarizes and classifies the nevi that will be reviewed both in part 1 (keratinocytic nevi) and in part 2 (nevi derived from adnexal structures) and can be used as a table of contents.
Epidermal Nevi Classified According to Morphological Criteria and Genes Implicated in Development.
| Gene involved | Locus | |||
|---|---|---|---|---|
| Keratinocytic nevi | Common keratinocytic nevus | FGFR3 | 4p16.3 | |
| PIK3CA | 3q26.32 | |||
| HRAS | 11p15.5 | |||
| NRAS | 1p13.2 | |||
| KRAS | 12p12.1 | |||
| FGFR2 | 10q26.13 | |||
| PENS | Unknown | Unknown | ||
| Epidermal nevus in Proteus syndrome | AKT1 | 14q32.33 | ||
| Type 2 segmental Cowden disease (PTEN) | PTEN | 10q23.31 | ||
| CHILD nevus | NSDHL | Xq28 | ||
| ILVEN | Unknown | Unknown | ||
| Epidermolytic epidermal nevus | KRT1 | 12.q13.13 | ||
| KRT10 | 17q21.2 | |||
| Acantholytic dyskeratotic epidermal nevus | ATPA2A | 12q24.11 | ||
| Nevi derived from adnexal structures | Nevus sebaceous | HRAS | 11p15.5 | |
| NRAS | 1p13.2 | |||
| KRAS | 12p12.1 | |||
| FGFR2a | 10q26.13 | |||
| Follicular nevi | Hair follicle nevus (congenital vellus hamartoma) | Unknown | Unknown | |
| Nevus comedonicus, Munro nevus | NEK9, FGFR2 | 14q24.3, 10q26.13 | ||
| Basaloid follicular hamartoma | Unknown | Unknown | ||
| Trichilemmal cyst nevus | Unknown | Unknown | ||
| Apocrine nevi | Apocrine nevus | Unknown | Unknown | |
| Syringocystadenoma papilliferum | BRAF | 7q34 | ||
| Eccrine nevi | Eccrine nevus | Unknown | Unknown | |
| Eccrine angiomatous hamartoma | Unknown | Unknown | ||
| Porokeratotic adnexal ostial nevus | GJB2 | 13q12.11 | ||
| Becker nevus | ACTB | 7p22.1 |
Abbreviations: HILD, congenital hemidysplasia, ichthyosiform erythroderma and limb defects; ILVEN, inflammatory linear verrucous epidermal nevus; PENS, papular epidermal nevus with skyline basal cell layer; PTEN, in this context, papillomatous, thick, epidermal, nonorganoid nevus.
Epidermal nevi often exhibit so-called organicity. This concept, in pathology, defines the simultaneous growth of several cell components in the same hamartoma. This phenomenon is often observed in hamartomas derived from adnexal structures and should not be confused with the concept, more often used in medicine, to define a symptom originating from organic and physical abnormality (as opposed to a psychological cause). Some texts classify epidermal nevi as organoid and nonorganoid (in practice, keratinocytic). Adnexal structures, for their part, can be the cause of certain lesions defined in some skin pathology text books as hamartomas,3 but which are, however, not considered nevi in most text books on clinical dermatology (for example, steatocystoma, fibrofolliculoma, and trichofolliculoma). We will not cover these lesions in the present review.
Pathophysiological BasisThe appearance of these lesions is due to genetic mutations or epigenetic changes that impact the expression of a cell clone during embryo development, leading to mosaicism, that is, the presence of 2 or more genetically distinct cell populations in the same individual.4 Four genetic mechanisms have been described that explain most cases of mosaicism in the skin:
- 1)
Somatic mutations in autosomal (dominant) genes that are lethal when they occur in the zygote but persist in the mosaic form. These mutations affect only one group of cells, which survive because they reside close to normal cells.5 Most epidermal nevi occur due to mutations of this type in 2 interlinked signaling pathways implicated in survival and cell proliferation: the phosphatidyl inositol 3 kinase (PI3K) pathway and the mitogen-activated protein kinase or MAP-kinase pathway.6
- 2)
Nonlethal mutations in autosomal genes that cause extensive skin disease when they occur in the zygote, but that can also become manifest in mosaic form if they are the result of a postzygotic mutation. These mutations can occur in an otherwise healthy individual, comprising an exclusively mosaic involvement (type 1 Happle mosaicism) or in a patient who already presents a generalized form of the disease, with a greater area of involvement, generally due to an additional mutation that leads to loss of heterozygosity (type 2 mosaicism).
- 3)
Mosaic mutations or epigenetic changes in genes linked to polygenic inflammatory diseases.
- 4)
Functional mosaicism linked to random inactivation of 1 of the X chromosomes in women, also known as lyonization.
Table 1 lists the genes whose mutation has been implicated in the pathogenesis of epidermal nevi. Recent discoveries in the field of genetics have shown that linking genotype to phenotype is far more complex than expected.7 The appearance of keratinocytic nevi may be due to mutations in up to 6 different genes, without any relationship with specific morphological findings. On the other hand, the same mutation in the same gene may give rise to morphologically distinct lesions, as occurs with mutations in RAS genes. Such mutations have been described in the pathogenesis of both keratinocytic and sebaceous nevi, even in the same patient.
Skin hamartomas follow a distribution pattern that depends on the type of cell from which they are derived.8,9 Most epidermal nevi follow the Blaschko lines (Happle pattern A and B archetype) with certain exceptions. Furthermore, the timing of the mutation and the onset of mosaicism determines the site and extent of the nevus. When the onset of mosaicism occurs towards the end of development, the lesions are limited and adopt different shapes9: an oval or triangular form is most frequent in epidermal nevi. The most extensive lesions result from mosaicism that occurred earlier and are more often associated with extracutaneous involvement. The epidermal nevus syndrome or Solomon syndrome was a term coined for certain patients in whom an epidermal nevus was associated with defects in the central nervous system, eyes, and/or bones.10 This terminology does not adequately describe the different entities that have been discovered in recent years and that are genetically and clinically very heterogeneous.11–14Table 2 summarizes the most important keratinocytic nevus syndromes described to date.
Some of the Best Characterized Syndromes Associated With Keratinocytic Nevi.
| Syndrome | Type of Nevus | Other Manifestations | Gene Involved (Transmission) | Reference |
|---|---|---|---|---|
| Epidermal nevus syndrome FGFR3 (García-Happle) | Keratinocytic nevus | Mental retardation, epilepsy | FGFR3 (sporadic) | 29,30 |
| CLOVES syndrome | Keratinocytic nevus | Lipomatous overgrowth, vascular, skeletal abnormalities | PIK3CA (sporadic) | 32 |
| PENS syndrome | Keratinocytic nevus (PENS) | Mild mental and psychomotor retardation that tends to improve with age, Achilles tendon shortening, hypospadia, curved penis | Unknown (most are sporadic, some familial cases with paradominant inheritance) | 46–48 |
| Proteus syndrome | Keratinocytic nevus | Limb hypertrophy, macrodactyly, vascular malformations, lipomas, cutis aplasia congenita and connective tissue nevus, lung disease, parotid adenoma, ovarian cystadenoma and breast cancer, endometrial cancer, testicular cancer | AKT1 (sporadic) | 50,51 |
| Type 2 segmental Cowden disease | Keratinocytic nevus (PENS) | Trichilemmomas, oral papillomas, fibromas, mucocutaneous neuromas, acral keratosis, genital lentiginosis, malformations, and vascular tumors and lipomas. Breast cancer, endometrial cancer, thyroid cancer, and colon cancer | PTEN (autosomal dominant) | 53,55,56 |
| CHILD syndrome | Keratinocytic nevus (CHILD nevus) | Ipsilateral skeletal aplasia or hypoplasia, punctiform calcifications in the bone ends, ipsilateral brain, lung, heart, or kidney defects | NSDHL (dominant X-linked, although the majority are sporadic) | 58 |
Abbreviations: CHILD, congenital hemidysplasia, ichthyosiform erythroderma and limb defects; CLOVES, congenital lipomatous overgrowth with vascular, epidermal and skeletal anomalies; PENS, papular epidermal nevus with skyline basal cell layer; PTEN, in this context, papillomatous, thick, epidermal, nonorganoid nevus.
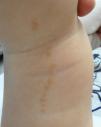
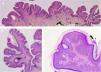
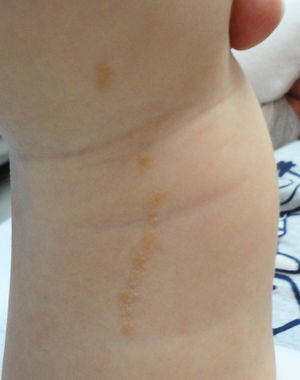
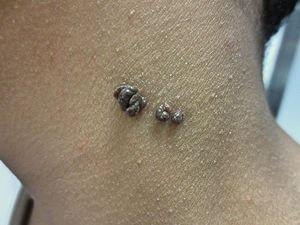
The common keratinocytic nevus presents as brown or grey plaques with a verrucous or velvety surface, often distributed along the Blaschko lines (Fig. 1). Their prevalence is between 0.1% and 0.5%. The lesions located in skinfolds can become moist and malodorous, whereas those on extensor surfaces often become dry and cracked. Some lesions may have a notably papillomatous appearance (Fig. 2). We often refer to these as verrucous nevi. The term nevus unius lateris refers to long linear keratinocytic nevi, generally on a limb. These are uncommon on the scalp and forehead, where sebaceous nevi are much more frequent. Some epidermal nevi occur in association with wooly hair on the scalp.15 Histologically, common keratinocytic nevi present acanthosis, papillomatosis, hyperkeratosis, thickening of the granular layer, and increased melanin in the basal layer (Fig. 3). Some histopathological variants have been described.16,17
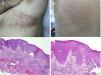
Verrucous nevus on the back of the neck. Histological image. A, Hematoxylin-eosin (HE) 2x: the epidermis shows acanthosis, papillomatosis, and hyperkeratosis. B, HE, 4x: detail of the papillomatous epidermis. C, HE, 10x: greater detail of the acanthosis, presence of hyperpigmentation of the basal layer, and loosely woven basket orthokeratotic hyperkeratosis.
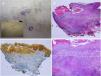
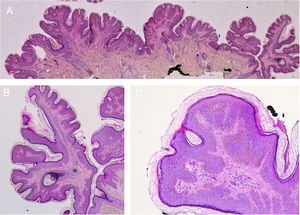
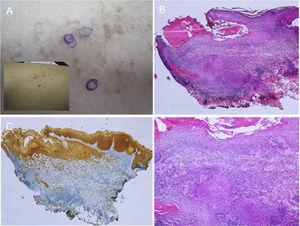
The appearance of malignant tumors on keratinocytic nevi is uncommon, although cases have been published (Fig. 4).18–24 They usually occur in adults. The most common is the development of squamous cell carcinoma. In addition, cases have been reported of basal cell carcinoma and porocarcinoma.
Squamous cell carcinoma developed over epidermic nevus, previously treated with electrocoagulation. A, Low resolution and detailed photograph. B, Hematoxylin-eosin (HE), 4x: 2 of the 3 lesions that were biopsied showed an epidermal proliferation and formation of strands that invaded the reticular dermis. C, Immunohistochemistry with AE1/AE3 cytokeratin, 4x: these strands of epithelial cells were invading the dermis. D, HE, 10x detail of the neoplastic proliferation composed of atypical keratinocytes that form keratin and horny pearls. Clinical images with permission of Dr. M. Concepción Sánchez Bermejo and Dr. María José González, of the Hospital de Manacor.
Common keratinocytic nevi are the result of mutations in up to 6 different genes, and so these may not translate into specific morphological features. All these are protooncogenes implicated in some types of cancer as well as in other benign acquired lesions such as seborrheic keratosis.25 We will now review the syndromes associated with keratinocytic nevi, based on their genetic classification.
Genetic Classification of Keratinocytic Nevi and Associated SyndromesIn approximately one third of common keratinocytic nevi, the same R248C heterozygous mutation in the FGFR3 oncogene can be found.25,26FGFR3 mutations are responsible for a relatively long list of syndromes and also neoplasms such as urinary bladder cancer, uterine cervical cancer, colorectal cancer, and spermatocytic seminoma. Although many cases of urinary tract cancer have been reported in patients with keratinocytic nevi,27,28 there is no proof to date that the nevus and neoplasm share the same mutation in any patients. In 2 patients with epidermal nevus and urothelial cancer in whom the FGFR3 gene was studied, both the hamartoma and neoplasm were wild type.29 Mosaic mutations in FGFR3 have been reported in some patients with mental retardation and epilepsy. When this occurs, the condition is known as the FGFR3 syndrome or García-Hafner-Happle syndrome.30,31
Another gene often affected is the PIK3CA gene. The same E545G mosaic mutation of PIK3CA has been implicated in keratinocytic nevi, seborrheic keratosis, and colorectal cancer.32PIK3CA mutations have also been associated with lipomatous growth and vascular and skeletal abnormalities, grouped in a syndrome known as congenital lipomatous overgrowth with vascular, epidermal and skeletal anomalies (CLOVES), Online Mendelian Inheritance in Man (OMIM) #612918.33 This syndrome was described in a series of 7 patients previously diagnosed with Proteus syndrome who had certain differential characteristics: progressive and mixed complex vascular malformations on the trunk, adipose tissue abnormalities, variable degrees of scoliosis, and elongated bones without progressive overgrowth and without bone distortion characteristic of Proteus syndrome.34 Subsequently, patients were described with malformations of the central nervous system and epilepsy.35 Unlike patients with Proteus syndrome, they did not develop connective tissue nevus (elastoma, collagenoma).36 Linear keratinocytic nevus is, in contrast, a characteristic lesion in CLOVES syndrome. The lesions tend to grow and become more verrucous until adolescence, and are stable thereafter.
Approximately 40% of keratinocytic nevi are the result of RAS mutations. In a study of 72 lesions, the HRAS gene was the most frequently affected, followed by NRAS and KRAS.37 RAS genes are important oncogenes and carry mutations in up to 30% of neoplasms in humans and in multiple syndromes (RASopathies). In the group of epidermal nevi, mosaic mutations in this group of genes may give rise to both keratinocytic nevi and organoid nevi and are an example of discordance between genotype and phenotype. Nevus marginatus, which has a central area rich in sebaceous glands and a more papular peripheral area with acanthosis and papillomatosis more similar to a keratinocytic nevus, is caused by mutations in RAS genes present in both components.38 Recently, the case has been reported of a girl with multiple nevoid lesions, some of which were consistent with keratinocytic nevus on the trunk and others with nevus sebaceous in the craniofacial region.39 The patient also showed delayed tooth development, cerebral arachnoid cyst, and optical atrophy. In both types of nevus, the same KRAS mutation was found. This mutation was not present in healthy skin or in peripheral blood of her parents. The syndromic forms associated with mutations in RAS genes will be covered in greater detail in the review of sebaceous nevus.
Some keratinocytic nevi with RAS mutations have been associated with the development of malignant neoplasms that carry the same mutation. This occurred for example in the case of a girl who presented a keratinocytic nevus and developed uterovaginal rhabdomyosarcoma at the age of 6 months.40 Both the hamartoma and the neoplasm showed the same KRAS mutation, which was not present in other tissues of the same patient. Another case is a 49-year-old man with extensive keratinocytic nevus who developed urothelial cancer in which the same mosaic HRAS mutation was detected in both lesions.41
Very recently, abnormalities have been described in the FGFR2 oncogene, whose mutation would explain 5% to 10% of keratinocytic nevi.42 The recent description of 2 fetuses with sebaceous nevi that carry mutations of this gene has been the source of debate, as some authors consider that these are keratinocytic nevi.43,44
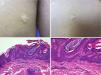
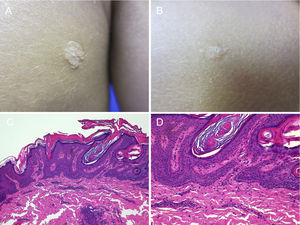
Other Keratinocytic Epidermal NeviPapular Epidermal Nevus with Skyline Basal Cell Layer (PENS) and PENS SyndromeIn 2011, Antonio Torrelo et al.45 described a new form of epidermal nevus with specific clinical and histological features, corresponding to solitary or multiple, congenital lesions or lesions with onset soon after birth, with a papular appearance and smooth or papillomatous surface, forming plaques that are rounded or with form of a comma, measuring 0.3 to 1.5cm with a random distribution. Only one case has been reported in which the lesions followed a Blaschkoid distribution.46 Histologically, they present regular epidermal acanthosis with thickened and rectangular epidermal crests, with orthokeratotic hyperkeratosis. The most characteristic finding is that the basal cell layer is arranged in a cobblestone pattern, with an empty eosinophilic region between the nuclei of the basal layer and the first keratinocytes of the stratum spinosum (Fig. 5).
Two PENS in the same patient. A and B, Two plaques, of 8mm and 5mm, with rounded form in the first case and polygonal somewhat linear form in the second. C, Hematoxylin-eosin (HE), 4x: biopsy showed epidermal hyperplasia with thickened and merged crests, with papillomatosis and compact hyperkeratosis. D, HE, 10x: at higher magnification, the basal cell layer in the epidermis can be seen with a cobblestone appearance.
PENS syndrome is manifest in the form of mental retardation and mild delay in development of psychomotor skills and epilepsy in the first year of life.47 These manifestations usually improve or even remit as the child gets older. Other findings include characteristic facies, shortened Achilles heel tendon, hypospadias, and curved penis.48,49
The genetic mutation responsible for this disorder and the mode of transmission are not known. In the original description, FGFR3 and PIK3CA mutations were ruled out.45 Generally, the lesions are sporadic, although families have been reported with more than one member affected.47,50
Epidermal Nevus in Proteus SyndromeProteus syndrome (OMIM #176920) is characterized by asymmetric tissue overgrowth caused by lethal, nonhereditary, mutations of the AKT1 gene that survive in mosaic form.51 The most characteristic changes are macrodactyly and hypertrophy of the limbs. Vascular malformations, lipomas, aplasia cutis congenita and connective tissue nevus, lung disease, parotid adenoma, ovarian cystadenoma and breast cancer, endometrial cancer, and testicular cancer may also be present. Up to 50% of patients present with epidermal nevi. These are flattened solitary or multiple plaques with a velvety surface, following the Blaschko lines. Histologically, acanthosis and hyperkeratosis are observed. Unlike other tumors associated with Proteus syndrome, epidermal nevus does not grow over time.52
Before the discovery of AKT1 mutations, Proteus-like syndrome due to mutations in the PTEN gene, with autosomal dominant transmission, has led to some confusion. Currently, it is thought that all these patients have Cowden disease along with an epidermal nevus (see the next section).53,54 All cases of Proteus syndrome are sporadic.
Type 2 Segmental Cowden Disease (PTEN nevus)Cowden syndrome (OMIM #158350) is a multisystemic disorder characterized by the appearance of multiple hamartomas and tumors, including breast cancer, endometrial cancer, thyroid cancer, and colon cancer. Tumors of the skin include trichilemmomas, oral papillomas, fibromas (including sclerotic fibroma or storiform collagenoma, which seems to be quite a specific finding), mucocutaneous neuromas, acral keratosis, genital lentiginosis, malformations, and vascular tumors and lipomas. It is produced by a mutation in the PTEN gene, a tumor suppressor gene of autosomal dominant transmission, although mutations have been reported in other genes.55
The lesion has also been called Cowden nevus or PTEN (used here as the acronym for papillomatous, thick, epidermal, nonorganoid nevus), and refers to the appearance of erythematous, hyperkeratotic and papillomatous papules that coalesce to form linear plaques following the Blaschko lines in patients with Cowden syndrome. It is caused by loss of heterozygosity in the PTEN gene (type 2 Happle mosaicism).54,56 It has also been called type 2 segmental Cowden disease,54 segmental overgrowth, lipomatosis, arteriovenous malformation and epidermal nevus syndrome.57 The lesion can be distinguished from the epidermal nevus characteristic of Proteus syndrome because of its more keratotic and papillomatous appearance and because it presents with other manifestations of Cowden syndrome.
CHILD Nevus and CHILD SyndromeCongenital hemidysplasia, ichthyosiform erythroderma, and limb defects (CHILD) syndrome (OMIM #308050) is characterized by an epidermal nevus in association with ipsilateral skeletal aplasia or hypoplasia, punctiform calcifications in bone ends, ipsilateral defects in the brain, lungs, heart, or kidney. It is transmitted according to an X-linked dominant pattern although most cases are sporadic. Clinical expression is very variable, even within the same family, and members may have very subtle lesions that hinder diagnosis.58 The disease arises as a result of mutation of the NSDHL gene,59 which participates in cholesterol metabolism. The mutation is very often lethal in male embryos, although cases have been described in males.60
CHILD nevus is often confused with an inflammatory disease, particularly psoriasis, in cases with more limited involvement.61 It presents as linear erythematous plaques or yellowish, scaling, psoriasiform or ichthyosiform plaques, following a Blaschkoid distribution. The most extensive lesions have a well-defined border at the midline. Interestingly, the great majority of patients described have involvement of the right half of the body. There is a particular predilection for folds (ptychotropism).62 Histologically, the disease is characterized by psoriasiform dermatitis with neutrophil exocytosis. In parakeratosis, it has been noted that the nuclei that persist are more rounded compared with those that are seen in psoriasis.61 We often see accumulations of histiocytes carrying lipid vacuoles that express CD68 and adipophilin in the papillary dermis, which under a papillomatous epidermis, may lead to suspicion of a verruciform xanthoma. These findings, in the context of a congenital Blaschkoid, lateral lesion, with ptychotropism, should lead to suspicion of CHILD syndrome.
Inflammatory Linear Verrucous Epidermal Nevus (ILVEN)This lesion is a linear plaque with an inflammatory appearance and erythema and scaling. It tends to present on the legs. In 25% of cases, the lesions are present at birth, a further 50% present during the first 6 months, and the remaining lesions can present up until the patient is 4 years of age. Histologically, we see a psoriasiform dermatitis, with epidermal hyperplasia and areas of parakeratosis on an epidermis without a granular layer, alternating with orthokeratosis over hypergranulosis. Areas with a more spongiotic appearance with lymphocyte exocytosis have been described. The disease can present in the form of type 1 or type 2 mosaicism in patients with generalized psoriasis.63 Several cases have been reported with good response to treatments for psoriasis (topical agents, phototherapy, systemic agents, biologics). Apart from an anecdotic association with arthropathy,64 extracutaneous manifestations have not been described in patients with linear epidermal verrucous inflammatory nevus. The genetic mutations responsible for the mosaicism giving rise to this nevus are not known, and, like psoriasis, it could be a polygenic disease.
Epidermolytic Keratinocytic NevusThis disease correponds to a mosaic form of epidermolytic ichthyosis or epidermolytic hyperkeratosis (OMIM #113800). It is due to dominant mutations in keratin 1 or 10 (KRT 1 o KRT10).65,66 These are somewhat pigmented verrucous lesions following a linear Blaschkoid distribution. They may be present at birth as a solitary lesion or multiple lesions, on any part of the body. The flexural lesions often become moist and malodorous. Histologically, they present hyperkeratosis, acanthosis, papillomatosis, and acantholysis in the granular layer. These findings are consistent with epidermolytic ichthyosis. Cases of type 2 mosaicism have been reported, with linear areas of greater involvement on an individual with this disease.67 Several families have been reported with parents affected with the nevoid form and children who present a complete form of epidermolytic ichthyosis.68–70 It is suspected that transmission occurs through gonadal mosaicism. In patients in whom this nevus is diagnosed, germinal involvement should be ruled out and genetic counselling made available.
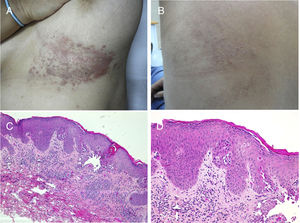
Acantholytic and Dyskeratotic Epidermal NevusAs in the previous nevus, most authors consider this nevus a mosaic form of a monogenic disease, although there is certain disagreement on this point. In this case, it would correspond to nevoid forms of Darier disease (OMIM #124200) or more rarely Hailey-Hailey disease (OMIM #169600). Numerous cases of segmental or linear Darier disease have been reported in the literature such as segmental or linear Darier disease.71–75 Clinically, these are linear keratotic crusty lesions that appear at puberty or later (Fig. 6). Exacerbations are characteristic and often associated with sweating and exposure to sunlight. Mucosal manifestations and lesions on the limbs, including palms, soles, and nails, characteristic of Darier disease, are not usually seen in nevoid forms. Furthermore, the lack of a family history of Darier disease is characteristic.
Segmental Darier disease. A and B, Clinical images of the submammary and left dorsal lesion in a female patient without any additional skin lesions. C, Hematoxylin-eosin (HE), 4x: biopsy showed epidermal acanthosis with minimal orthokeratotic hyperkeratosis, suprabasal acantholysis, and formation of an intraepidermal blister. D, HE 10x: at higher magnification, the dyskeratotic changes can be appreciated better, with presence of rounded bodies and spots. The patient was diagnosed with segmental Darier disease.
Histologically, we see the presence of a hyperkeratotic and parakeratotic epidermis in which we observe intraepidermal cleavage that contains acantholytic and dyskeratotic keratinocytes, with presence of corps ronds and grains (Fig. 6). In some cases of acantholytic and dyskeratotic epidermal nevus, a mutation has been found in ATP2A2, characteristic of the disease.76,77
Some authors affirm, however, that acantholytic and dyskeratotic epidermal nevus and segmental Darier disease are not the same entity.78–80 The examples presented are congenital lesions (instead of lesions developing after puberty), in which mutation in the ATP2A2 gene has not been demonstrated or it is possible to confirm expression of SERCA2 (the expression product of ATP2A2) by immunohistochemistry.
Nevoid forms of Hailey-Hailey disease are rare. One case has been published of type 1 segmental involvement,81 and another case with presentation at 3 months of age with a relapsing linear lesion that subsequently developed symmetric lesions characteristic of the disease.82 In this patient, loss of heterozygosity was demonstrated in the area affected earliest (type 2 mosaicism).83 Histologically, suprabasal acantholysis is observed with lymphocytes and eosinophils. Dyskeratosis can be present, although it is not usually as evident as in Darier disease and rounded bodies or grains are not usually observed. Diagnosis of Hailey-Hailey disease usually requires immunofluorescence to rule out an autoimmune blistering disease, particularly, pemphigus vulgaris.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Garcias-Ladaria J, Rosón MC, Pascual-López M. Nevus epidérmicos y síndromes relacionados. Parte 1: nevus queratinocíticos. Actas Dermosifiliogr. 2018;109:677–686.