Maki et al.,1 previously published a case of dipeptidyl peptidase-4 inhibitor (DPP4i)-induced bullous pemphigoid (BP) and concurrent acquired reactive perforating dermatosis (ARPD). We recently observed a similar case in a diabetic patient.
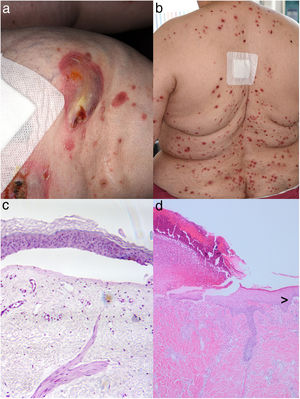
A 69-year-old female attended with an 8-week history of severely itching skin lesions. On examination, several tense blisters on her knees and generalized partly umbilicated papules with a central adherent keratotic plug were observed (Fig. 1a and b). No blistering was evident in the latter lesions. Results of histopathological examination have also been detailed in Fig. 1c and d. Direct immunofluorescence showed linear deposits of IgG and C3 at the basement membrane zone. Indirect immunofluorescence (IIF) using salt-split skin showed band-like IgG deposits at the epidermal side. Enzyme-linked immunosorbent assay revealed highly elevated antibodies against BP-180 (NC16A) with 140U/ml (<20); BP-230 and desmoglein 1 and 3 were within the normal range. Her co-morbidities included hypertension, obesity, and diabetes mellitus (type II), which was treated with vildagliptin 50mg/metformin 850mg twice daily for 6 months. The first BP lesions developed 4 months after starting therapy with vildagliptin. A diagnosis of DPP-4i-induced BP (DPP4i-BP) with concurrent ARPD was made. We recommended to substitute vildagliptin and initiated systemic dimetindene maleate (4mg twice daily) and prednisolone 150mg in a tapering dosage regimen. Under this management the patient improved.
Showing crusty burst blisters on the right knee (a) and multiple papules with central dark plugs on the back (b) of a diabetic patient under DPP4i treatment. Histopathological examination of a skin biopsy specimen taken from the right knee revealed the presence of sub-epidermal bullae containing some perivascular lymphocytic infiltrates without eosinophils (c). Histology obtained from a papulous lesion on the upper back partly revealed transepidermal elimination of necrotic basophilic collagen bundles into a cup-shaped epidermal depression, whereas the here shown figure is dominated by ulceration (d; hematoxylin–eosin stain, magnification 200×). Interestingly, the border of a sub-epidermal blister formation is also seen (>; hematoxylin–eosin stain, magnification 100×).
Large studies including meta-analyses provided strong evidence that DPP4i, in particular vildagliptin, are frequently the trigger of BP onset which is a relatively common autoimmune blistering disease of the elderly.3,4 Detailed observations have revealed that DPP4i-BP tend to show rather a non-inflammatory phenotype, with less erythema than BP without DPP4i intake.1–3 Moreover, the histological picture frequently shows only sparse inflammatory infiltrates (e.g., eosinophils).1–4 It has also been demonstrated that the HLA-DQB1*03:01 genotype is often associated with DPP4i-BP.2,3 DPP4 – a synonym for CD26 – is expressed in a variety of cells such as T lymphocytes and other immune cells.3,4 Hence, one may expect that DPP4 inhibition could induce alterations in immunological processes, including the development of epitopes for DPP4i-BP autoantibodies. DPP4i-BP auto-antibodies may also target epitopes on BP-180 that are distinct from those of DPP4i-unrelated BP (NC16A) and can be more precisely detected by full-length BP-180 ELISAs.1–3 As summarized by Maki et al.,1 the association of DPP4i-unrelated BP and ARPD has previously been reported in sporadic cases.1 In all 5 cases, onset of ARPD preceded the onset of BP.1 Pathogenesis of ARPD is barely understood. Lesions are characteristically within the reach of scratching (Fig. 1b) and can be reproduced experimentally by scratching the skin.5 Researchers have proposed that ARPD represents a cutaneous reaction to superficial trauma due to scratching. ARPD is extremely frequently associated with diabetes mellitus/nephropathy leading to the idea that scratching may cause micro-trauma and necrosis of epidermal/dermal structures, probably due to diabetic microangiopathy.5 ARPD-associated scratching and skin trauma may contribute the realization of DPP4i-BP.2–4
In conclusion, we reported an unusual clinical constellation in a DPP4i-treated diabetic patient who simultaneously developed BP as well ARPD. Since the use of DPP4i is very frequent worldwide, more such cases are likely to occur.
Conflict of interestThe authors declare not to have any conflict of interest.