Cutaneous myiasis is a group of diseases characterized by the infestation of the skin of vertebrate animals—humans or other animals—by dipteran larvae.
Following the anatomical classification of myiasis, 1 based on several previous proposals, cutaneous myiasis can be categorized into the following entities:
- •
Wound myiasis: parasitization of the surface of exposed wounds.
- •
Cutaneous myiasis: involves the penetration of dipteran larvae into healthy skin, which can be further subcategorized into:
- ∘
Furuncular cutaneous myiasis: the larva creates a cavity connected to the entry point, without distant movement, where it develops part of its life cycle (fig. 1).
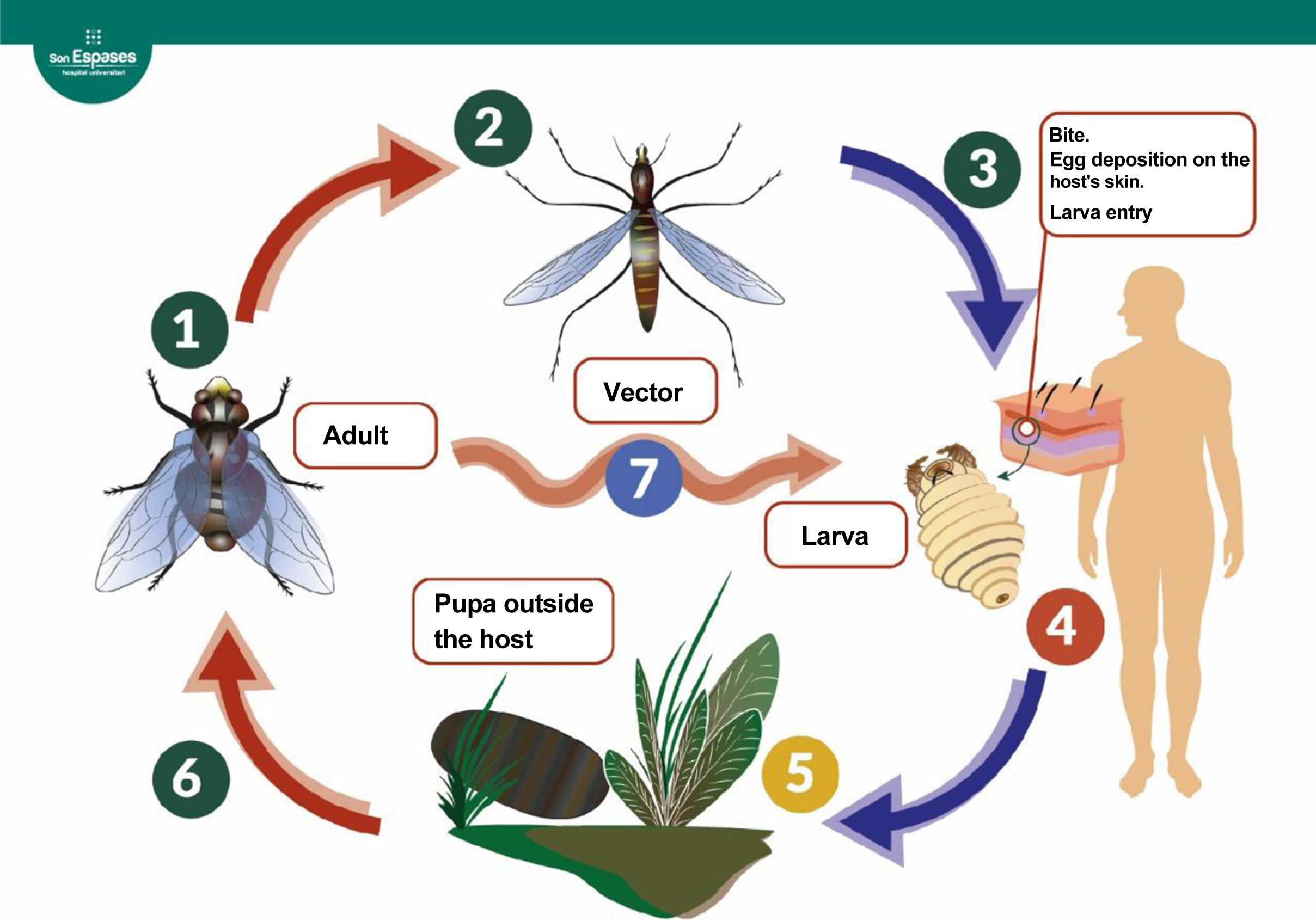
Figure 1.Life cycle of the dipterans responsible for furuncular myiasis.3 The adult fly lays eggs (1). In the case of Dermatobia hominis, the eggs are attached to the surface of an arthropod vector (2), which acts as a vector when it bites a mammal, accidentally depositing the eggs on it (3). Other species (7), including the genera Cochliomyia, Cuterebra, and Wohlfahrtia, can lay the eggs directly on the host or on surfaces that come into direct contact with the host (genus Cordylobia) without the need for a vector. The eggs hatch upon contact with the host's warmth. The larva penetrates the skin, creating a cavity where it develops over the following weeks by feeding on the host's tissues (4). The mature larva leaves the host, forming a pupa outside (5). After about a month, a new adult specimen emerges (6).
- ∘
Migratory or progressive cutaneous myiasis: occurs due to the migration of the larva under the skin surface.
- •
Cavitary or organic myiasis, which is named after the natural cavity or organ it affects, with the most frequently described in the literature being ophthalmomyiasis, oral, nasal, urogenital, cerebral, and intestinal myiasis.
Focusing on furuncular cutaneous myiasis, the most widely involved species are Dermatobia hominis and Cordylobia anthropophaga, the former being the main cause in Central and South America, and the latter in Africa.
Both species are characterized by their larval stage, exhibiting radial spines on the anterior segments and a spiracle that allows them to breathe on the posterior segment.2 Proper identification of the causative agent requires studying the anatomical characteristics of the complete specimen—shape, spines, spiracle, pigmentation—by an experienced microbiologist or entomologist.
The usual clinical presentation of this condition consists of the appearance of an inflammatory nodule about 2cm in diameter with a central opening, located at the site of egg inoculation. It is often associated with itching and even a sensation of movement in the lesion. Occasionally, the larval channel may be less obvious if covered by a crust of necrotic debris and larval waste products. Generally, the lesions are solitary, occupied by a single larva, although cases of multiple infestations have been reported. In the case of Dermatobia, the most common presentation is a single lesion associated with an arthropod bite that acted as a vector for the eggs in exposed areas of the skin, such as limbs or the head, while Cordylobia usually presents as multiple lesions in unexposed areas such as the back or buttocks.4
Diagnosis requires a high degree of clinical suspicion, based on history-taking and physical examination.
Dermoscopy can be useful, as it allows visualization of the larva's posterior spiracles when it is close to the surface. Llamas-Velasco et al.5 propose keeping the dermatoscope on the lesion for several minutes, forcing the larva to move toward the surface by depriving it of oxygen.
In doubtful cases, another useful tool is skin ultrasound,6 with which a 1cm to 1.5cm oval, echogenic mass can be observed under the epidermis, with an acoustic shadow, internal Doppler vascularization, and spontaneous movement, allowing the identification of the number of larvae and their distribution inside the cavity.
Case reportA 10-year-old man, with no past medical history, had traveled to Costa Rica a month before the consultation. During his stay, he suffered several insect bites, one of them on the scalp, which evolved over the following days into a 1.5cm inflammatory and pruritic nodule. Upon returning to Spain, the lesion developed a central opening and the child started exhibiting inflammatory adenopathies in the retroauricular and occipital regions remaining afebrile at all times. The lesion was initially assessed by his pediatrician and interpreted as an abscessed bite and treated with topical antibiotics without improvement. Oral amoxicillin-clavulanic acid was added, and drainage was attempted without yielding any content. After 4 weeks of disease progression without improvement, the patient was referred to the Dermatology Department for reevaluation, at which point, given the highly suggestive clinical presentation and epidemiological context of myiasis, we decided to confirm our diagnostic suspicion.
Description of the techniqueWe describe a simple and cost-effective exploratory technique for identifying this condition (see video of the supplementary data). It involves occluding the opening of the larval channel with petroleum jelly for, at least, 30minutes, which reduces the oxygen supply to the larva. After this occlusion period, petroleum jelly is removed, and the larva is immediately observed ascending to the surface to breathe.
Treatment consists of extracting the larva, and in some cases, as with our patient, surgical enlargement of the exit opening is necessary to allow complete removal of the specimen without fragmentation.
Several extraction methods are described in the literature,7 such as simple pressure around the opening to force the larva out, using forceps to grasp the specimen if it appears on the surface, or surgical removal. The choice should be based on the type of larva and its maturation stage. For instance, species with anchoring structures like spines, specimens in advanced maturation stages, or large larvae relative to the exit opening are more difficult to remove using non-surgical methods.8
In the case of Dermatobia hominis infestation, manual pressure around the lesion is often insufficient for extraction purposed, as we have confirmed in our routine clinical practice, which is consistent with the findings made by Francesconi and Lupi.1 These authors also propose physical occlusion of the exit opening to force the larva out, although they note the risk of suffocating the specimen inside the lesion, making extraction more difficult with the corresponding inflammatory response and formation og foreign body granuloma if not removed.
Oral ivermectin has been described in literature for cases of multiple infestations or in immunocompromised individuals,9 always with larval removal to avoid the consequent foreign body inflammatory response. As a preventive measure, the use of insect repellents and long, tight-fitting cotton clothing is advised when traveling to endemic areas.3,4
IndicationsPatients with a furuncle with a central opening and a history of travel to tropical regions of Latin America in the preceding weeks, especially if they report insect bites.
ComplicationsThe technique has no complications or contraindications.
Regarding complications of the condition per se, bacterial superinfection is the most common one. In general, the larva does not migrate from the inoculation site, and there is no invasion of deeper tissues, although cases of fatal cerebral myiasis in children have been reported due to penetration through open fontanelles.3
Conclusions- •
Myiasis caused by Dermatobia hominis should be suspected in patients with a furuncular lesion, especially if a central opening is observed, and recent travel to tropical areas of Latin America.
- •
We describe a simple and affordable diagnostic technique for furuncular myiasis with high specificity, based on the occlusion of the opening with petroleum jelly to create an anoxic environment for the larva and promote its exit.
- •
After larval extraction, short-term follow-up is advised to ensure healing of the residual lesion.
None declared.
Conflicts of interestNone declared.