Dermoscopy allows us to visualize structures and colors in pigmented lesions that are not visible to the naked eye. By comparing serial dermoscopic images of a mole, we can appreciate changes occurring long before any instability would be apparent on evaluation of conventional macroscopic images. One of the primary objectives of digital dermoscopy is to facilitate the storage and later comparison of serial images of atypical moles.1 Instability in such moles, as evidenced by increasing atypia, may give rise to a suspicion that the lesion is an incipient melanoma or a mole evolving towards melanoma.
There are basically 4 possible scenarios in the diagnosis of a melanoma using dermoscopy. The first of these is the need to establish a morphologic differential diagnosis between a possible melanoma and a nonmelanocytic lesion, such as a seborrheic keratosis or basal cell epithelioma.2 In this case, the differential diagnosis is strictly morphologic. The greater diagnostic accuracy of dermoscopy compared to the unaided eye will help to prevent many unnecessary or unnecessarily urgent biopsies and excisions. And, more importantly, the use of dermoscopy will help to prevent the inadequate or delayed treatment of a melanoma.
The second scenario occurs when we use a dermoscope to observe a pigmented lesion that clearly exhibits the clinical features of either a melanoma or an ordinary benign mole.2 In such cases, the diagnostic value of the technique is low, but the educational value is very high. When physicians first use a dermoscope it is very important that they become familiarized with and can distinguish the morphological features in moles and melanomas that can be easily identified. Later they will need to look for the same structures and colors in atypical moles and incipient melanomas, where their presence may be much harder to discern and their diagnostic value much greater.
This second scenario allows us to introduce another reflection that is very important to the central theme of the present article: What are the features that make it easy to diagnose a large superficial spreading melanoma with the naked eye? How and why does a large superficial spreading melanoma acquire the morphological features that are so characteristic of such lesions (ABCDE—asymmetry, border irregularity, color variegation, diameter greater than 6mm, and evolution)? The clinical appearance of any large melanoma is neither accidental nor arbitrary, but rather determined by the underlying genetic alterations in the tumor cells in conjunction with the anatomic and microenvironmental characteristics of the area of skin where the lesion develops. In our opinion, there are 3 fundamental biological processes that must be taken into account in this context, as described in a number of published articles.3–6 The 3 processes that directly influence the morphology of most large superficial spreading melanomas are uncontrolled proliferation/loss of senescence, genetic instability, and regression.
Acquired benign moles never reach the size attained by large late stage melanomas. The defining characteristic of such melanomas is not the speed of their proliferation, which is very variable, but rather the fact that—unlike the case of benign moles—their growth is not self-limiting in space or over time. It has been proposed that a mechanism of cellular senescence may be at least partly responsible for the self-limiting growth of moles.7 In the case of melanomas, this mechanism is either absent from the outset (in the case of de novo melanoma) or disappears from the lesion at a certain point (in the case of a melanoma arising from a melanocytic nevus).
Genetic instability is one of the principal mechanisms responsible for the intratumor heterogeneity involved in the progression of many malignant tumors.8 Such instability contributes to the asymmetric morphology of large melanomas,9 which are characterized by variations in color and texture within the lesion and the presence of raised or ulcerated areas. In such cases, a clinical diagnosis is generally very simple. Genetic instability also leads to the appearance of tumor cell subpopulations with varying metastatic efficiency and differing responses to available treatments, a situation that represents a therapeutic challenge.8
Focal evidence of regression can be observed in many large melanomas. It is well established that melanoma is a rather immunogenic tumor.10 The immune system frequently attacks and partially destroys the melanoma, and in large melanomas this process is readily apparent to the naked eye and facilitates diagnosis. Occasionally, the primary melanoma is completely destroyed, although less immunogenic subclones capable of producing metastases may be selected during the process.
While many other biological processes characteristic of the malignant tumor phenotype are likely to influence the appearance and evolution of melanomas and many other tumors,11 each one of the 3 processes discussed above has an immediate and undeniable morphological signature in melanoma. Thus, it is of great diagnostic value to be able to detect these features at a very early stage in a melanocytic tumor. If any signs of the presence of these characteristics are detected, the lesion must be evaluated with particular care. The advantage of investigating the lesion with dermoscopy rather than the naked eye is obvious, as we shall see below. The features that can be easily seen with the unaided eye in a large melanoma are not usually obvious in an incipient melanoma or in a dysplastic and unstable nevus, and this difference has important consequences for the prevention and early diagnosis of melanomas.
The third situation in which we advocate the use of dermoscopy in the diagnosis of melanoma poses more difficulties. The problem arises when we have to make a differential diagnosis between an atypical mole and an incipient melanoma because, as we know, to the naked eye some atypical moles appear more atypical than some incipient melanomas.12 The difficulty is even greater in patients who have many atypical moles. In this setting, dermoscopy has been shown to increase our diagnostic accuracy, although it does not offer 100% accuracy. Numerous algorithms have been developed to improve the diagnosis of melanoma using dermoscopy in conjunction with pattern analysis.2 Typically these algorithms evaluate the presence or absence of a series of specific morphologic findings in the lesion. The results obtained with an algorithm can provide guidance on whether or not the lesion is a melanoma and can help us to decide whether a particular lesion should be excised or monitored? None of the algorithms have demonstrated 100% sensitivity and the fact that several algorithms of this type have been developed is, in itself, an indication that none of them are entirely satisfactory: all of them fail to diagnose certain melanomas and all of them lead to the removal of benign atypical moles. While some of these algorithms take into account information contributed by the patient concerning the evolution of the lesion,13,14 most are based solely on the lesion's morphology (structures and colors) as revealed by dermoscopy.2 The algorithms are used in conjunction with a still photograph of the lesion. What these algorithms take into account is the presence or absence of certain morphologic features that can be observed with the dermoscope and not any evolutive significance that might be inferred from these morphologic findings or even the evolution itself.
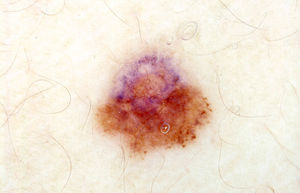
The difficulty of correctly classifying certain problematic lesions as either benign or malignant using manual dermoscopy brings us to the fourth scenario, in which the decision to monitor or extirpate an atypical mole of uncertain malignant potential is determined primarily by monitoring its evolution over time. This is the scenario that calls for digital dermoscopy.1 We know that in some cases the malignant potential of a melanoma with no obvious morphological features characteristic of malignancy is only recognized because of unexpected changes observed over a few months of follow-up. Similarly, many incipient melanomas are easily diagnosed on the basis of changes observed with digital dermoscopy over longer follow-up periods, when such changes may not yet be apparent to the naked eye. In any case, the information provided by manual dermoscopy can also be of use in this scenario. The biological processes mentioned above (uncontrolled proliferation/loss of senescence, genetic instability, and regression) often have very clearly defined dermoscopic correlates.3 In many atypical moles and incipient melanomas, the correct interpretation of the morphologic findings can indicate the dynamic of change even when we are only looking at a single still image taken at the time the lesion was observed through the dermoscope. There is some overlap between the third and fourth scenarios if we are able to capture the information about the evolution of the lesion that can be deduced from a single dermoscopic image (Fig. 1).
We have enumerated 3 biological processes (uncontrolled proliferation/loss of senescence, genetic instability, and regression) that are primarily responsible for the morphology of large superficial spreading melanomas. Do these processes have obvious dermoscopic correlates in very early melanomas and in atypical and unstable moles characterized by increasing atypia? In our opinion, they clearly do.
The characteristic marker of proliferation in dermoscopy is the presence of globules at the periphery, sometimes in the form of clumps or pseudopods and at others forming a more linear pattern (fingerlike radial projections). A ring of regularly distributed peripheral globules is characteristic of many growing compound melanocytic nevi.15 In Spitz-Reed nevi a star burst pattern may develop. However, certain findings should make us consider the possibility of malignant potential or evolution towards malignancy, including, for example, irregularity in the size, color, and distribution of the peripheral globules, a focal pattern (Fig. 1), or abnormally intense pigmentation. The presence of peripheral globules should raise suspicion when a lesion, because of its size, should not have globules or when globules are observed in patients who, on account of their age, should not have growing moles. We must also pay particular attention when peripheral globules reappear in a mole in which they had previously disappeared or when they appear for the first time.
Dermoscopy provides many indications of genetic instability in melanocytic lesions, and most of the algorithms used heavily weight structural asymmetry (Fig. 1) and an abundance of structures and colors.2 It is reasonable to conclude that marked asymmetry and/or the presence of a multicomponent pattern are the result of the emergence of melanocyte subclones that interact in different ways with the skin microenvironment. Even manual dermoscopy can provide an indication of this phenomenon. Monitoring with digital dermoscopy can make such indications much more evident in many early melanomas and in unstable atypical moles characterized by growing atypia. When the process is clearly evident and progressive, monitoring of the lesion must be concluded and appropriate action taken.
Regression is also readily apparent on dermoscopy, appearing as a pepper-like pattern of gray dots (Fig. 1) or an area of scar-like whitish depigmentation. In flat lesions the presence of a blue-whitish veil is also usually associated with areas of regression. Regression should not be confused with the physiological involution that produces a loss of pigmentation in many moles over time. Regression is the result of an attack by the immune system on a benign or malignant melanocytic neoplasm, probably triggered by the recognition of premalignant or malignant changes.10 In the case of conventional melanocytic nevi with halos (Sutton nevi), depigmentation is caused by a dysfunction of the immune system and there is generally no need to remove the lesions. In the case of atypical moles displaying regression, the problem is the lesion. Regression is, by its very nature, a dynamic and progressive phenomenon (until the immune system has eliminated whatever triggered the attack or until the attack is neutralized by immune system evasion mechanisms set in motion by the tumor).10 Lesions with obvious dermoscopic regression are rare. In such cases we favor removal of the lesion.
Pathological tumor angiogenesis is a fourth process that is generally not visible to the unaided eye in early or incipient melanomas but is easily recognized with dermoscopy. The visualization of vessels within a melanocytic tumor is not synonymous with malignancy. Comma-shaped vessels, sometimes very abundant and prominent, are also found in intradermal nevi. However, certain vascular patterns (dotted, corkscrew, and polymorphous vessels, among others) are highly suggestive of malignancy.16 The presence of an inflammatory response in the tumor microenvironment (another of the basic biological processes involved in tumor progression11) can also give rise to erythema and increased vascularity. The pathological increase in vascularization within a melanoma is often focal or irregularly distributed. In some hypomelanotic or amelanotic melanomas, vascularization may be the only diagnostic key.
The 4 processes outlined in this article (uncontrolled proliferation/loss of senescence, genetic instability, tumor regression, and pathological tumor angiogenesis) are, by definition, dynamic processes with well-defined dermoscopic correlates in many cases. The presence in a melanocytic tumor of the dermoscopic findings usually associated with these biological processes helps us to recognize where the lesion has come from and where it is going, even on the basis of a single dermoscopic image. Digital dermoscopy is a very useful tool when we want to evaluate the stability of an atypical mole over time and to verify that it is not becoming more atypical.1 But the use of dermoscopy may be questioned when the image of an atypical melanocytic lesion already reveals elements indicating that it is highly probable that the lesion is changing and becoming more atypical. In such cases should we monitor the lesion or remove it? In the last case described above, we favor excision. As other authors have indicated, what is important in such cases is not a correct diagnosis but rather a correct decision on the appropriate course of action.17
The great majority of atypical moles are stable or change without increasing atypia. Monitoring such lesions with digital dermoscopy corroborates this fact and prevents unnecessary excisions.1 But some moles, which may or may not be clinically atypical at the outset, evolve to become increasingly atypical and eventually give rise to melanoma. The interpretation of certain dermoscopic findings from a biological standpoint is a good way to investigate these lesions and to identify changes characteristic of malignancy or progression towards malignancy. Excision of clearly unstable lesions can contribute not only to an earlier diagnosis of melanoma but also to the prevention of melanoma when we remove a mole that is in the process of becoming malignant. We should act very selectively to avoid any unnecessary increase in the removal of benign moles. The effectiveness of our management should be revealed by a reduction in the incidence of melanoma in the long term among patients being monitored.18
As a practical approach, and starting from a biological standpoint, our proposal is to initially evaluate the presence of structural asymmetry in atypical moles. If asymmetry is observed in the absence of any other suspicious finding, the lesion should be monitored since many acquired atypical moles and quite a few congenital melanocytic nevi display asymmetry along one axis but are stable in the long term. If the structural asymmetry—a feature that can be indicative of genetic instability—is associated with signs of uncontrolled proliferation/loss of senescence, regression, or pathological angiogenesis, we always favor removal of the mole, even when the findings do not yet suggest that it has become a melanoma. Since certain melanomas arise from melanocytic nevi, we can prevent some of them from developing when we are monitoring melanocytic nevi and detect a lesion showing signs of problematic instability. The preventive potential of dermoscopy should not lead us to remove many more atypical moles, but rather allow us to remove moles that display obvious signs of instability or increasing atypia and lesions undergoing biological processes characteristic of tumor progression toward malignancy.
Our approach has certain limitations. In facial lentigo and acral lentiginous melanoma, the initial dermoscopic findings are usually determined by the peculiar arrangement of the tumor melanocytes produced by the microanatomy of the skin in these sites.19 Early follicular invasion in lentigo maligna produces an appearance of asymmetric perifollicular pigmentation. Small acral lentiginous melanomas display a parallel ridge pattern produced by the accumulation of tumor melanocytes along the deep intermediate ridges penetrated by the intraepidermal spiral ducts or acrosyringia. These findings can provide the key to diagnosis in incipient cases of these melanoma subtypes before the appearance of the signs indicative of the four biological mechanisms described above.
Finally, rapidly growing nodular melanomas also reveal the limitations of our approach. Early diagnosis of such lesions is both difficult and of critical importance in reducing the mortality associated with this melanoma subtype. Many such lesions fulfill only one of the criteria for suspicious lesions defined by the ABCDE rule: evolution or change (E). To facilitate their early diagnosis, some authors have proposed a rule based on elevation, firmness to palpation, and continued growth for more than a month (the EFG rule).20 Dermoscopy of incipient nodular melanomas very often does not yield very much information although it may provide some data that will be useful in reaching a diagnosis, for instance, a prominent and atypical vascular pattern reflecting the neoangiogenesis characteristic of such tumors, which is much more obvious in hypomelanotic lesions.20 The detection of these melanomas is greatly aided by monitoring of high-risk patients using baseline panoramic images. These images are also useful for patient self-monitoring and constitute one of the most accurate ways of detecting new, rapidly growing small or unstable lesions, which can be amelanotic. Such lesions should always be evaluated immediately and very carefully. When there is any doubt, the best course is to remove the lesion.
Please cite this article as: Pizarro Á, Santiago J, Santiago D. Prevención y diagnóstico precoz del melanoma con dermatoscopia: una perspectiva biológica. Actas Dermosifiliogr. 2015;106:3–6.