Dermoscopy is a complementary technique that has led to major advances in the diagnosis of pigmented skin lesions. The aim of this study was to describe the dermoscopic features of a series of melanomas and analyze the differences between melanomas in situ and invasive melanomas.
Material and methodsWe retrospectively recorded epidemiological, clinical, histologic, and dermoscopic features of a series of 200 primary melanomas. We performed a descriptive and analytical study of the dermoscopic features identified.
ResultsThe mean age of the patients was 63 years and there was a similar distribution of male and female patients. The most common histologic subtypes were superficial spreading melanoma (62.5%) and lentigo maligna (25.5%); 67% of the melanomas had a Breslow thickness of less than 1mm and 24.5% were melanomas in situ. Overall,the most common global dermoscopic features were the multicomponent pattern (33.5%), the reticular pattern (18%), and the nonspecific pattern (15.5%). The most common local features were structureless homogeneous areas (67.5%), white-blue structures (58%), an atypical pigmented network (55.5%), and irregularly distributed dots and globules (44%). The following features were more common in invasive melanomas than in melanomas in situ: blue, gray, red and white colors, multicomponent and homogeneous patterns, dots and globules, blue-white structures, homogeneous areas, a blue-white veil, white shiny structures, a reverse pigment network, and milky-red areas. The reticular pattern was more common in melanomas in situ.
DiscussionThe use of dermoscopy has contributed to the early diagnosis of melanoma. The most common dermoscopic features of melanoma are multiple structures and colors (multicomponent pattern), an atypical reticular pattern (with wide, irregular meshes), and an absence of distinguishing features (nonspecific pattern) associated with the presence of vascular structures.
ConclusionsDermoscopy facilitates the diagnosis of melanoma and could be useful for differentiating between melanoma in situ and invasive melanoma.
La dermatoscopia es una técnica complementaria que ha supuesto un gran avance en el diagnóstico de las lesiones pigmentadas. El objetivo del presente trabajo es describir las características dermatoscópicas de una serie de melanomas y analizar las diferencias entre los melanomas in situ y los melanomas invasivos.
Material y métodosSe obtuvieron de forma retrospectiva los datos referentes a las características epidemiológicas, clínicas, histológicas y dermoscópicas de una serie de 200 melanomas primarios. Se realizó un estudio descriptivo y analítico de las variables dermoscópicas.
ResultadosLa edad media de los pacientes fue de 63 años, con una distribución similar por sexos. Los tipos histológicos más frecuentes fueron el melanoma de extensión superficial (62,5%) y el lentigo maligno (25,5%). El 67% de los melanomas tuvieron un índice de Breslow menor de 1mm y un 24,5% fueron melanomas in situ. Los patrones dermatoscópicos globales más frecuentes fueron el multicomponente (33,5%), el reticular (18%) y el inespecífico (15,5%). Las estructuras dermatoscópicas más frecuentes fueron las áreas homogéneas desestructuradas (67,5%), las estructuras blanco-azuladas (58%), el retículo pigmentado atípico (55,5%) y los puntos y glóbulos de distribución irregular (44%). Los colores azul-gris, rojo y blanco, los patrones multicomponente y homogéneo, los puntos y glóbulos, las estructuras blanco-azuladas, las áreas homogéneas, el velo azul-blanquecino, las estructuras blancas brillantes, el retículo invertido y las áreas rojo lechosas fueron más frecuentes en los melanomas invasivos que en los melanomas in situ. El patrón reticular fue más frecuente en los melanomas in situ.
DiscusiónEl uso de la dermatoscopia ha contribuido al diagnóstico precoz del melanoma. Los datos dermatoscópicos más frecuentes en el melanoma son la presencia de múltiples estructuras y colores (patrón multicomponente), un patrón reticular atípico con una red ensanchada e irregular y la ausencia de criterios dermatoscópicos (patrón inespecífico) asociada a la presencia de estructuras vasculares.
ConclusionesLa dermatoscopia facilita el diagnóstico de melanoma. Podría tener utilidad para diferenciar los melanomas in situ de las formas invasivas.
Pigmented lesions are a common reason for consultation in routine clinical practice, and the number of patients consulting for these lesions has multiplied in recent years thanks to melanoma prevention campaigns and other factors. Melanoma in situ has a better prognosis than invasive melanoma, and therefore early diagnosis results in longer survival and lower morbidity and mortality. The ABCD (Asymmetry, Border irregularity, Color, Diameter) algorithm or rule has been used in clinical practice for years,1 but it is not adequate for pigmented lesions and does not always distinguish between melanocytic and nonmelanocytic lesions. Furthermore, it is not always reliable in the evaluation of small lesions.2 Dermoscopy, which is a widely used, cheap, and simple technique, has led to enormous advances in the diagnosis of pigmented skin lesions. Dermoscopes are equipped with a conventional or polarized light source that allows lesions to be studied at a higher magnification and are associated with a 10% to 30% improvement in diagnostic accuracy in clinical settings.3,4 The aim of the current study was to describe the dermoscopic features of a series of melanomas and determine which features are associated with melanomas in situ and which are associated with invasive melanomas.
Material and MethodsWe performed a retrospective study of 200 primary cutaneous melanomas diagnosed at the dermatology department of Hospital General Universitario Gregorio Marañón in Madrid, Spain; all the tumors had been examined by dermoscopy prior to surgical excision between January 1, 2007 and December 31, 2009. The diagnosis was confirmed by histology in all cases. The preoperative diagnosis was established using both clinical criteria (ABCD rule) and dermoscopic criteria, using the standardized two-stage diagnostic procedure proposed by the virtual Consensus Net Meeting on Dermoscopy.5 All the dermoscopic images were taken using the DermLite dermoscope (magnification ×10) and evaluated by 2 operators (CCB and JAAI). Note was also taken of epidemiological characteristics (patient sex, age), clinical features (skin phototype and size and location of the melanoma), and histologic features (subtype, Breslow thickness, and Clark level).
Statistical AnalysisWe performed a descriptive study of the dermoscopic features of the melanomas analyzed and investigated their association with epidemiological, clinical, and histologic variables. The χ2 test was used to test for associations between qualitative variables. When this was not possible (i.e., for variables with few cases), we simply examined correlations to test for possible differences. Statistical significance was set at a P value of less than .05. The data were analyzed using descriptive and analytical techniques in SPSS, version 13.0 for Windows.
ResultsEpidemiological, Clinical, and Histologic FeaturesThe epidemiological, clinical, and histologic features are summarized in Table 1.
Epidemiological, Clinical, and Histologic Features.
| No. | % | |
| Sex | ||
| Female | 110 | 56.4 |
| Male | 85 | 43.6 |
| Skin phototype | ||
| I | 0 | 0 |
| II | 152 | 78 |
| III | 43 | 22 |
| IV | 0 | 0 |
| History of sunburn | ||
| Yes | 116 | 59.5 |
| No | 79 | 40.5 |
| Tumor site | ||
| Scalp | 10 | 5 |
| Face | 52 | 26 |
| Neck | 5 | 2.5 |
| Chest | 10 | 5 |
| Abdomen | 10 | 5 |
| Back | 53 | 26.5 |
| Upper extremities | 20 | 10 |
| Palms | 0 | 0 |
| Lower extremities | 26 | 13 |
| Soles | 8 | 4 |
| Nails | 3 | 1.5 |
| Genital mucosa | 2 | 1 |
| Oral mucosa | 1 | 0.5 |
| Histologic subtype | ||
| Superficial spreading melanoma | 125 | 62.5 |
| Lentigo maligna melanoma | 51 | 25.5 |
| Nodular melanoma | 10 | 5 |
| Acral lentiginous melanoma | 8 | 4 |
| Animal-type melanoma | 2 | 1 |
| Desmoplastic melanoma | 1 | 0.5 |
| Not specified | 3 | 1.5 |
| Tumor thicknessBreslow thickness | ||
| Melanoma in situ | 49 | 24.5 |
| <1mm | 85 | 42.5 |
| ≥1 and <2mm | 32 | 16 |
| ≥2 and <4mm | 27 | 13.5 |
| >4mm | 7 | 3.5 |
| Clark level | ||
| Clark I | 49 | 24.5 |
| Clark II | 43 | 21.5 |
| Clark III | 34 | 17 |
| Clark IV | 64 | 32 |
| Clark V | 10 | 5 |
| Histologic ulceration | ||
| Yes | 12 | 6 |
| No | 188 | 94 |
| Associated nevus | ||
| Yes | 20 | 10 |
| No | 180 | 90 |
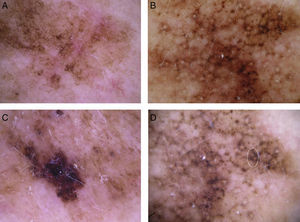
The most common global dermoscopic features were the multicomponent pattern (33.5%), the reticular pattern (18%), the nonspecific pattern (15.5%), and the homogeneous pattern (7%) (Table 2 and Fig. 1). The other dermoscopic features identified are shown in detail in Table 2 and Fig. 2. The specific dermoscopic features of lentigo maligna melanoma (LMM) are shown in Table 3.
Dermoscopic Features of Melanomas in our Series.
| Dermoscopic Structure | No. | % |
| Color | ||
| Black | 178 | 89 |
| Dark brown | 186 | 93 |
| Light brown | 173 | 86.5 |
| Blue-gray | 135 | 68 |
| White | 54 | 27 |
| Red | 59 | 29.5 |
| Pattern | ||
| Reticular | 36 | 18 |
| Multicomponent | 67 | 33.5 |
| Nonspecific | 31 | 15.5 |
| Homogeneous | 14 | 7 |
| Starburst | 5 | 2.5 |
| Globular | 4 | 2 |
| Parallel | 4 | 2 |
| Pigmented network | 114 | 57 |
| Typical | 3 | 1.5 |
| Atypical | 111 | 55.5 |
| Dots and globules | 88 | 44 |
| Regular | 0 | 0 |
| Irregular | 88 | 44 |
| Projections | 26 | 13 |
| Regular | 2 | 1 |
| Irregular | 24 | 12 |
| Pigment stains | 75 | 37.5 |
| Central | 25 | 12.5 |
| Peripheral | 50 | 25 |
| White-blue structures | ||
| Present | 116 | 58 |
| Absent | 84 | 42 |
| White scarring | ||
| Present | 39 | 19.5 |
| Absent | 161 | 80.5 |
| Blue-white veil | ||
| Present | 44 | 22 |
| Absent | 156 | 78 |
| Homogeneous areas | ||
| Present | 75 | 37.5 |
| Absent | 125 | 62.5 |
| White shiny structures | ||
| Present | 22 | 11 |
| Absent | 178 | 89 |
| Reverse pigment network | ||
| Present | 19 | 9.5 |
| Absent | 181 | 90.5 |
| Vessels | ||
| Linear irregular vessels | 36 | 18 |
| Dotted vessels | 17 | 8.5 |
| Hairpin vessels | 8 | 4 |
| Comma vessels | 6 | 3 |
| Corkscrew vessels | 6 | 3 |
| Glomerular vessels | 1 | 0.5 |
| Arborizing vessels | 1 | 0.5 |
| Milky-red areas | 52 | 26 |
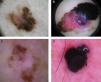
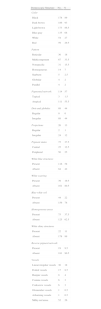
Global dermoscopic patterns. A, Superficial spreading melanoma with a Breslow thickness of 0.5mm showing a reticular pattern. B, Superficial spreading melanoma with a Breslow thickness of 2.4mm showing a multicomponent pattern. C, Superficial spreading melanoma with a Breslow thickness of 1.7mm and a nonspecific pattern showing asymmetrical structures. D, Nodular melanoma with a Breslow thickness of 2.3mm showing a homogeneous pattern.
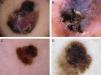
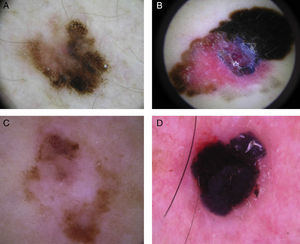
The most common dermoscopic features in the melanomas in our series. A. Structureless, homogeneous pigment areas at the periphery of the lesion (ovoids). B, White-blue structures and homogeneous pigment areas. C, Thick, irregular atypical pigment network. D, Multiple irregularly distributed dots and globules.
Dermoscopic Features of Lentigo Maligna Melanoma.
| Dermoscopic Features | No. | % |
| Asymmetric follicular openings | 23 | 45.1 |
| Blue-gray dots and globules | 31 | 60.8 |
| Annular-granular pattern | 24 | 47.1 |
| Rhomboidal structures | 25 | 49 |
| Homogeneous areas | 21 | 41.2 |
| Isobar structures | 13 | 25.5 |
| Increased vascular network | 21 | 41.2 |
| Reddish rhomboidal structures | 10 | 19.6 |
| Darker lesion by dermoscopy | 11 | 21.6 |
Table 4 shows the association between epidemiological, clinical, histologic, and dermoscopic features of the melanomas by histologic subtype. In the case of acral melanomas, it is worth noting that we observed a parallel crest pattern in lesions on the soles of the feet and a linear irregular pattern in lesions affecting the nail. Several features were more common in invasive melanomas than in melanomas in situ (P<.05), namely, blue, gray, red and white colors, the multicomponent and homogeneous or structureless patterns, irregularly distributed dots and globules, blue-white structures, a blue-white veil, white shiny structures, a reverse pigment network, and milky-red areas (Table 5). The reticular pattern and the characteristic asymmetrically pigmented follicular openings seen in lentigo maligna melanoma were significantly more common in melanomas in situ than in invasive melanomas (P<.05).
Epidemiologic, Clinical, Histologic, and Dermoscopic Features of Melanoma by Histologic Subtype.
| Superficial Spreading Melanoma, % (No.) | Lentigo Maligna Melanoma, % (No.) | Nodular Melanoma, % (No.) | Acral Lentiginous Melanoma, % (No.) | |
| Sex | ||||
| Male | 68.3 (56) | 19.5 (16) | 8.5 (7) | 3.7 (3) |
| Female | 61.6 (69) | 31.3 (35) | 2.7 (3) | 4.5 (5) |
| History of sunburn | ||||
| Yes | 63.2 (79) | 62.7 (32) | 50 (5) | 0 |
| No | 36.8 (46) | 31.3 (19) | 50 (5) | 100 (8) |
| Tumor site | ||||
| Head and neck | 10.4 (13) | 96 (49) | 20 (2) | 0 |
| Trunk | 55.2 (69) | 2 (1) | 30 (3) | 0 |
| Extremities | 32 (40) | 2 (1) | 50 (5) | 100 (8) |
| Breslow thickness | ||||
| ≤1mm | 69.6 (87) | 82.3 (42) | 0 | 37.5 (3) |
| >1mm | 31.4 (38) | 17.7 (9) | 100 (10) | 62.5 (5) |
| Breslow thickness | ||||
| <1mm | 71.2 (89) | 82.4 (42) | 0 | 37.5 (3) |
| ≥1 and <2mm | 20 (25) | 9.8 (5) | 10 (1) | 12.5 (1) |
| ≥2 and <3mm | 6.4 (8) | 5.9 (3) | 30 (3) | 12.5 (1) |
| ≥3 and <4mm | 3.4 (3) | 0 | 40 (4) | 0 |
| >4mm | 0 | 2 (1) | 20 (2) | 37.5 (3) |
| Colors, No. | ||||
| ≤2 | 9.6 (12) | 39.2 (20) | 30 (3) | 37.5 (3) |
| >2 | 90.4 (113) | 60.8 (31) | 70 (7) | 62.5 (5) |
| Global pattern | ||||
| Reticular | 28 (35) | 0 | 0 | 0 |
| Globular | 3.2 (4) | 0 | 0 | 0 |
| Homogeneous | 4.8 (6) | 0 | 40 (4) | 0 |
| Starburst | 4 (4) | 0 | 0 | 0 |
| Parallel | 0 | 0 | 0 | 60 (3) |
| Multicomponent | 46.4 (58) | 6 (3) | 30 (3) | 25 (2) |
| Nonspecific | 14.4 (18) | 15.7 (8) | 30 (3) | 25 (2) |
| Dermoscopic structures | ||||
| Atypical network | 82.4 (103) | 0 | 20 (2) | 0 |
| Irregular dots | 61.6 (77) | 7.8 (4) | 40 (4) | 25 (2) |
| Irregular projections | 16.8 (21) | 2 (1) | 0 | 0 |
| Irregular pigment | 36 (45) | 3.9 (2) | 10 (1) | 12.5 (1) |
| Blue structures | 70.4 (80) | 21.6 (11) | 90 (9) | 50 (4) |
| White areas | 25.6 (32) | 9.8 (5) | 20 (2) | 0 |
| Homogeneous areas | 45.6 (57) | 15.7 (8) | 60 (6) | 12.5 (1) |
| Blue veil | 25.6 (32) | 3.9 (2) | 70 (7) | 0 |
| White shiny structures | 16 (20) | 0 | 10 (1) | 0 |
| Reverse network | 14.4 (18) | 0 | 10 (1) | 0 |
| Milky-red areas | 28.8 (36) | 15.7 (8) | 60 (6) | 12.5 (1) |
Epidemiological, Clinical, Histologic, and Dermoscopic Features of Melanoma in Situ and Invasive Melanoma.
| Melanoma in Situ | Invasive Melanoma | Statistical Significance | |
| Sex | P=.492 | ||
| Male | 37.2% (19) | 44.3% (67) | |
| Female | 58.8% (30) | 55.6% (84) | |
| Tumor site | |||
| Scalp | 2 (20%) | 8 (80%) | |
| Face | 20 (38.5%) | 32 (61.5%) | |
| Neck | 3 (60%) | 2 (40%) | |
| Chest | 2 (20%) | 8 (80%) | |
| Abdomen | 0 | 10 (100%) | |
| Back | 13 (24.5%) | 40 (75.5%) | |
| Upper extremities | 3 (15%) | 17 (85%) | |
| Lower extremities | 6 (23.1%) | 20 (76.9%) | |
| Soles | 0 | 3 (60%) | |
| Nails | 0 | 5(100%) | |
| Other | 0 | 6 (100%) | |
| Sunburn | P<.05 | ||
| Yes | 69.3% (34) | 57% (86) | |
| No | 30.6% (15) | 43% (65) | |
| Histologic subtype | |||
| Superficial spreading melanoma | 49% (24) | 66.8% (101) | |
| Lentigo maligna melanoma | 51% (25) | 17.2% (26) | |
| Nodular melanoma | 0 | 6.6% (10) | |
| Acral lentiginous melanoma | 0 | 5.2% (8) | |
| Other | 0 | 3.9% (6) | |
| Color | |||
| Black | 34.7% (17) | 47.7% (72) | |
| Dark brown | 87.8% (43) | 94.7% (143) | |
| Light brown | 89.8% (4) | 85.4% (129) | |
| Blue-gray | 40.8% (20) | 76.2% (115) | P<.05 |
| White | 16.3% (8) | 30.5% (46) | P<.05 |
| Red | 14.3% (7) | 34.4% (52) | P<.05 |
| Global pattern | |||
| Reticular | 28.5% (14) | 14.5% (22) | P<.05 |
| Globular | 2% (1) | 2% (3) | |
| Homogeneous | 4.1% (2) | 7.9% (12) | P<.05 |
| Starburst | 2% (1) | 2% (3) | |
| Parallel | 0 | 2.6% (4) | |
| Multicomponent | 14.3% (7) | 39.7% (60) | P=.001 |
| Nonspecific | 10.2% (5) | 17.2% (26) | P=.238 |
| Dermoscopic structures | |||
| Atypical pigment network | 44.8% (22) | 59% (89) | |
| Irregular dots and globules | 30.6% (15) | 48.3% (73) | P<.05 |
| Irregular projections/pseudopods | 8.2% (4) | 13.2% (20) | |
| Irregular pigment stains | 8.2% (4) | 30.5% (46) | P<.05 |
| Blue-white structures | 30.6% (15) | 69.4% (101) | P<.05 |
| White areas | 16.3% (8) | 20.5% (31) | |
| Homogeneous areas | 20.4% (10) | 43% (65) | P<.05 |
| Blue-white veil | 2% (1) | 28.5% (43) | P<.05 |
| White shiny structures | 2% (1) | 13.9% (21) | P<.05 |
| Reverse network | 4.1% (2) | 11.3% (17) | |
| Vascular structures | |||
| Milky-red areas | 12.2% (6) | 30.5% (46) | P<.05 |
| Linear irregular vessels | 8.2% (4) | 21.2% (32) | P<.05 |
| Dotted vessels | 0 | 11.3% (17) | P<.05 |
| Hairpin vessels | 2% (1) | 4.6% (7) | |
| Comma vessels | 2% (1) | 3.3% (5) | |
| Corkscrew vessels | 0 | 4% (6) | |
The diagnosis of thin melanomas (<1mm) has increased in recent years, probably partly due to the combined effects of melanoma prevention campaigns and the use of dermoscopy.6–8 Melanomas tend to have multiple colors, with most lesions showing 3 or more colors when examined by dermoscopy.9 The multicomponent pattern is the most common dermoscopic feature described in most studies of melanoma.10–12 This pattern has the strongest association with melanoma (odds ratio of 4.3) and the detection of different colors and structures should therefore alert to a possible diagnosis of malignancy.5,10–14 The reticular pattern in melanomas is an atypical pattern, with a broad, irregular mesh, typically seen at the periphery of the lesion. Subtle changes in the characteristics of the pigment network can help to establish a diagnosis of early-stage melanoma.15 Melanoma should also be suspected when faced with a nonspecific pattern (absence of dermoscopic features) combined with red colors or multiple vascular structures. The nonspecific pattern has been observed in between 7% and 15% of melanomas, depending on the series.10,16 Lesions exhibiting this pattern should be excised for histologic examination.
Homogeneous areas were the most common dermoscopic feature in our series of melanomas, and have been described in between 65% and 80% of melanomas in other series.10,12 They are areas of diffuse pigmentation, with no other identifiable structures. The distribution of these structureless areas is important, as a regular diffuse distribution is associated with benign melanocytic lesions, while an irregular, focal distribution is more characteristic of malignant lesions.5,10,11
In our series, white-blue structures were more common in invasive melanomas than in melanomas in situ. These structures correspond to regression areas and are frequently associated with melanoma, although they may also be seen in certain benign lesions with regression. Most experts, however, recommend the excision of lesions with white-blue structures occupying over 10% of the tumor area.17–20
The pigment network is the most characteristic dermoscopic feature of melanocytic lesions.5 The thickness of the network lines, together with size and spacing of the mesh holes, helps to differentiate between the typical pigment network (thin, uniformly spaced mesh) seen in benign melanocytic lesions, such as junctional nevi and solar lentigines, and the atypical pigment network (thick, prominent lines and irregular holes) seen in malignant melanocytic lesions. The atypical pigment network was observed in over 50% of melanomas in our series, which is very similar to previously reported rates.10,12 The atypical network is the most common dermoscopic finding in melanomas in situ, and is key for the diagnosis of very early stage melanoma. Slight changes in the pigment network of thin melanomas can be the first dermoscopic sign of a possible diagnosis of melanoma.15
Irregularly sized and distributed dots and globules are observed by dermoscopy in up to 44% of melanomas, but all the dots and globules in our series had a uniform size and distribution. These irregular structures are observed in both benign and malignant melanocytic lesions, and their distribution (regular or irregular), location (in the center or at the edge of the lesion), and color all play an important role in determining the nature of the lesion.11,12
Other structures included in many of the diagnostic algorithms for melanoma, such as the blue-white veil and radial streaming, were less common in our series. The blue-white veil was observed in 22% of lesions, and was more common in invasive melanomas than in melanomas in situ. This veil has been described as being highly specific for melanoma,5 but it may also be seen in other nonmelanocytic lesions such as basal cell carcinoma.22,23 Furthermore, the proportion of lesions exhibiting this sign varies considerably from one study to the next, suggesting possibly that it is a somewhat subjective marker, characterized by high interobserver variability and a lack of reproducibility.5,10,12 Nevertheless, it is important to correctly interpret this sign, as it has been particularly linked to invasive melanomas. Radial streaming was uncommon in our series, and the projections were irregularly distributed in all cases. Most cases were observed in superficial spreading melanomas. Radial streaming and pseudopods are an important dermoscopic finding for the diagnosis of melanocytic lesions.11,21 Regularly distributed projections at the periphery of the lesion, giving rise to what is known as the starburst pattern, are a characteristic feature of Reed/Spitz nevus, while irregular or asymmetric projections are highly suggestive of melanoma in the radial growth phase.
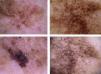
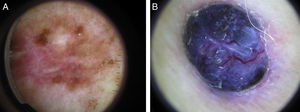
Sixty percent of the melanomas in our series had vascular patterns (Fig. 3). Milky-red areas and linear irregular and dotted vessels were more common in invasive melanomas than in melanomas in situ. All the vascular structures identified were more common in nodular and su-perficial spreading melanomas. Vascular structures can be difficult to identify and may be missed in more heavily pigmented lesions due to the presence of other structures. Dotted and linear irregular vessels are the most common vessels seen in melanomas, and invasive melanomas in particular.24,25 The presence of these vessels, together with milky red areas, should raise suspicion of melanoma.
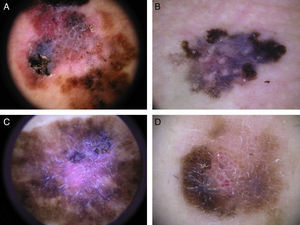
Melanoma of the face has several distinctive dermoscopic features not seen in other types of melanoma (Fig. 4).26–29 In our series, at least 1 of the 4 Stolz criteria was present in 81% of LMMs. We did not detect any dermoscopic features associated with benign lesions, such as milia-like cysts, yellow opaque areas, or light brown fingerprint-like structures. Asymmetrically pigmented follicular openings were more common in melanomas in situ than in invasive melanomas of the face. Based on the Stolz progression model for LMM, these openings would be the earliest dermoscopic signs of facial melanoma. In the next stage, blue-slate-gray dots would join to form short lines that eventually form rhomboidal structures. Finally, atypical melanocytes would invade the hair follicles and the rhomboidal structures would become thicker and more prominent until the follicular openings were completely obliterated; this stage is viewed as homogenous pigment areas on dermoscopy.26 The presence of a single Stoltz criterion is not sufficient for the diagnosis of malignancy, as these criteria can also be seen in benign lesions. Of the 4 criteria, asymmetrically pigmented follicular openings and rhomboidal structures have been described as being more characteristic of LMM and are rarely found in other pigmented lesions.11,29 Blue-gray dots may also be observed in other pigmented lesions of the face.28 They correspond to areas of regression and can be found in all lesions that exhibit histologic regression. One distinguishing feature is that the annular-granular pattern and blue-gray dots tend to be more homogeneous and regular in these more “benign” lesions than in LMM. Additionally, these lesions do not have asymmetrically pigmented follicular openings or isobar structures, features that correspond to the invasion of hair follicles by atypical melanocytes. These dermoscopic findings may be useful for distinguishing between LMM and other pigmented lesions of the face.
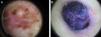
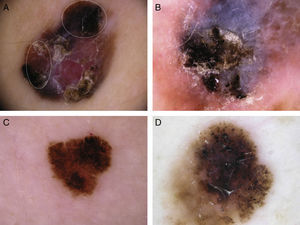
Typical dermoscopic features of lentigo maligna melanoma of the face. A, Asymmetrically pigmented follicular openings and annular-granular pattern. B, Rhomboidal structures. C, Homogeneous facial areas. D, Target structures corresponding to the invasion of the hair follicles by atypical melanocytes (ovoid).
Acral lentiginous melanoma was uncommon in our study. The most common global patterns were the parallel crest pattern in palmoplantar melanoma and the linear irregular pattern in melanoma of the nail. Our findings are similar to previous reports of acral lentiginous melanoma in the literature.30
Several dermoscopic structures were more common in invasive melanomas than in melanomas in situ (Fig. 5). Blue-gray, red, and white colors, a combination of these colors, and the multicomponent pattern were more common in invasive melanomas than in melanomas in situ. The most common local dermoscopic finding in melanomas in situ was the atypical pigment network. All the other dermoscopic features were found in just a small proportion of melanomas in situ. Irregularly distributed dots and globules, white shiny structures, homogeneous areas, the reverse pigment network, white-blue structures, the blue-white veil, and vascular patterns and structures were more common in invasive melanomas that in melanomas in situ.
One of the limitations of this study is a possible lack of objectivity in interpreting the dermoscopic findings as this was a retrospective study in which there was a known histologic confirmation of melanoma in all cases. Furthermore, some lesions that were not examined by dermoscopy prior to surgical excision and histologic study (because of a low or nonexistent suspicion of melanoma) may have been excluded.
In conclusion, dermoscopy permits the visualization of structures that can facilitate the clinical diagnosis of pigmented lesions. While melanoma must always be confirmed histologically, we identified certain dermoscopic features that were more common in invasive melanomas than in melanomas in situ that could be used as a complementary diagnostic tool.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their consent.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ciudad-Blanco C, Avilés-Izquierdo JA, Lázaro-Ochaita P, Suárez-Fernández R. Hallazgos dermoscópicos para la detección precoz del melanoma. Análisis de 200 casos. Actas Dermosifiliogr. 2014;105:683–693.