Thalidomide is the treatment of choice for severe or recurrent erythema nodosum leprosum. Its use has been associated with deep vein thrombosis in patients with blood disorders, however, particularly when used in combination with corticosteroids or chemotherapy. We describe a case of deep vein thrombosis in a 43-year-old man with lepromatous leprosy who was being treated with thalidomide and prednisone for a type 2 leprosy reaction (erythema nodosum leprosum); the patient also had transiently positive antiphospholipid antibody results. We stress the importance of considering deep vein thrombosis, a potentially fatal complication, in dermatology patients treated with thalidomide.
La talidomida es el fármaco de elección en el tratamiento del eritema nodoso leproso severo/recurrente. Su uso ha sido relacionado con trombosis venosas profundas (TVP) en pacientes con enfermedades hematológicas (especialmente cuando se le asocia con corticoides y quimioterapia).
Presentamos un caso de TVP en un hombre de 43 años, con lepra lepromatosa en tratamiento con talidomida y prednisona por una leprorreación tipo 2 (eritema nodoso leproso), con anticuerpos antifosfolípidos positivos transitorios.
Resaltamos la importancia de tener en cuenta esta posible complicación, potencialmente fatal, en pacientes tratados con talidomida por enfermedades dermatológicas.
Leprosy, or Hansen disease, is a chronic granulomatous infection caused by Myobacterium leprae. It mainly affects cool parts of the body, such as the skin, the upper airways, the anterior segment of the eye, the superficial segments of the peripheral nerves, and the testicles. Mycobacterium lepromatosis sp. nov is a new, recently identified, mycobacterium1 that causes diffuse lepromatous leprosy (or diffuse leprosy of Lucio and Latapo2). This form of leprosy is endemic in Mexico and the Caribbean.
Leprosy can be classified as paucibacillary (when no acid-fact bacilli are found in tissues or smears) or multibacillary (when 1 or more acid-alcohol-fast bacilli are found in tissues or smears). According to the guidelines of the World Health Organization, paucibacillary leprosy should be treated with a combined regimen of sulfone and rifampicin for 6 months, whereas multibacillary leprosy should be treated with sulfone, rifampicin, and clofazimine for a year. Other bactericidal antibiotics for infections caused by M leprae are available for patients with refractory or recurrent disease or for patients who do not respond to conventional treatment or who have sulfone intolerance. Examples are fluoroquinolones, minocycline, and clarithromycin.
Patients with leprosy can develop acute, immune-mediated reactions, known as type 1 (reversal) reactions or type 2 reactions (erythema nodosum leprosum).
Thalidomide is the treatment of choice for severe or recurrent erythema nodosum leprosum. Its use, however, has been linked to serious complications such as deep vein thrombosis,3 teratogenicity, neuropathy, and hyperkalemia.
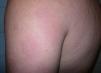
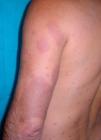
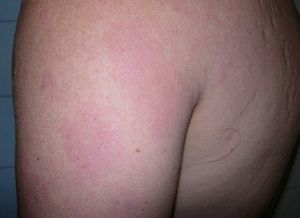
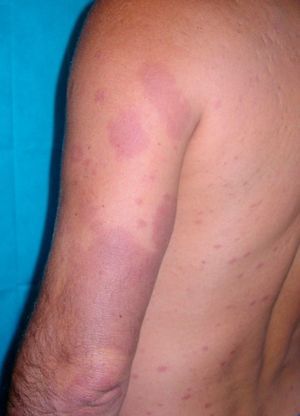
Case DescriptionWe report the case of a 43-year-old Brazilian man who consulted in 2008 for skin lesions of 3 years’ duration. The lesions were painful, well-demarcated, slightly infiltrated erythematous plaques with a loss of touch sensation (Fig. 1); they were located on the limbs, the arms, the buttocks, and the trunk. Skin biopsy showed interstitial granulomatous dermatitis with a moderate superficial perivascular lymphocytic infiltrate and aggregates of histiocytes at the interface between the reticular and the papillary dermis. Ziehl-Neelsen staining showed small numbers of acid-alcohol-fast bacilli, arranged singly and in clumps (globi). The mucus and lymph study was positive (bacteriological index 2+ and morphological index of 40%), and electromyography showed a mild to moderate sensory-predominant axonal polyneuropathy. The initial diagnosis was borderline lepromatous leprosy (although this was later revised to lepromatous leprosy) and treatment was initiated with rifampicin 600mg/d, clofazimine 50mg/d (plus 300mg on the first day of the month), and sulfone 100mg/d. Six months after starting treatment, the patient presented with new lesions, worsening of existing lesions, and fever of 38°C. A second skin biopsy showed a predominantly perivascular and periadnexal granulomatous reaction with foamy histiocytes that spared the epidermis. Ziehl-Neelsen staining showed abundant bacilli. Although the significance of these findings was initially unclear, the case was diagnosed as a type 1 leprosy reaction and treatment with prednisone 30mg/d was initiated. The lesions, however, continued to worsen (Fig. 2) and new lesions suggestive of erythema nodosum leprosum appeared. In view of this new situation, the patient was started on thalidomide (up to 150mg/d) with gradual tapering of the corticosteroid dose. Two months after starting treatment with thalidomide, the patient presented with swelling and pain in the right leg and Doppler ultrasound revealed an infrapopliteal deep vein thrombosis. The laboratory tests showed normal complete blood count and biochemistry and coagulation profiles but slightly elevated antiphospholipid antibody levels: immunoglobulin (Ig) A anti-β2-glycoprotein I antibodies (anti-β2GPI), 34.0 U (normal value, <20 U); IgG antiphosphatidylserine antibodies, 33.6 U (normal value, <16.0 U); IgG anticardiolipin antibodies (aCL), 27.70 IgG phospholipid units (GPL)/mL (normal value, <25.00 GPL/mL), and normal IgM aCL levels. Thalidomide was withdrawn and anticoagulation treatment was initiated with enoxaparin followed by acenocoumarol. The tests were repeated after 8 weeks, with negative or normal results in all cases.
DiscussionThe presence of antiphospholipid antibodies has been linked to numerous infectious diseases, both viral (human immunodeficiency virus infection, hepatitis C virus infection, and parvovirus B19 infection) and bacterial (syphilis, malaria, and leprosy); the antibodies are generally transient and can disappear with treatment.4 The presence of aCL and anti-β2GPI antibodies has been reported in 42.7% (range, 21%-67%) and 44.8% (range, 2.9%-89%), respectively, of patients with lepromatous leprosy or borderline lepromatous leprosy.5 One study observed that aCL antibody positivity was more common in patients with multibacillary leprosy (80.7%) than in those with paucibacillary leprosy (8.3%) and that titers were not affected by treatment.6
The presence of aCL, lupus anticoagulant, or anti-β2GPI is considered to be independent of the risk of thrombosis, and antiphospholipid syndrome (APS) is only considered to exist in a patient with vascular thrombotic events or complications of pregnancy if a positive test result is obtained for 1 of these antibodies on 2 or more occasions at least 12 weeks apart.7
Our patient had IgA anti-β2GPI, which are not included in the classification criteria for APS. Even if they were, however, the criteria would not apply as these antibodies were detected on only 1 occasion.
Most studies indicate that antiphospholipid antibodies in infections are mainly of the IgM isotype,8 but Loizou et al.4 found that IgA aCL were more common in patients with leprosy. The matter is somewhat controversial as many authors have reported that infection-related antiphospholipid antibodies are not associated with procoagulant states.9 In autoimmune disorders, such as systemic lupus erythematous and primary APS, the binding of antiphospholipid antibodies to phospholipids is enhanced by the cofactor β2GPI. Nonthrombogenic antiphospholipid antibodies, however, do not require β2GPI to enhance their binding to phospholipids. Two types of antiphospholipid antibodies are distinguished based on this difference: autoimmune (or β2GPI-dependent) antibodies and infection-associated (or β2GPI-independent) antibodies. The line between the 2, however, is somewhat blurred.
Akerkar et al.10 described a patient with borderline tuberculoid leprosy, gangrene, and elevated IgM aCL titers, while Wallin et al.11 described the case of a patient with lepromatous leprosy who developed bilateral fibular artery obstruction due to the presence of aCL and lupus anticoagulant, confirming that these antibodies can provoke thrombotic events.
Thalidomide used alone in patients with blood dyscrasias has not been associated with a higher rate of thrombotic events (deep vein thrombosis incidence of 1%). When the drug is used in association with other agents, however, such as dexamethasone or chemotherapy agents, the risk increases (up to 10%), particularly in patients with neoplastic disease (metastatic renal cell carcinoma, myelodysplastic syndrome, and multiple myeloma).3 All the studies published to date have shown that the risk of thrombotic events in patients receiving thalidomide is highest in the first 2 months of treatment, an observation that is consistent with our case. In a study of 25 patients with skin diseases treated with thalidomide (100-300mg/d) alone, 5 patients (20%) developed deep vein thrombosis and 4 of these had erythema nodosum leprosum.12
In our review of the literature, we found 4 patients with erythema nodosum leprosum treated with thalidomide and corticosteroids who developed deep vein thrombosis.13–16 One of the patients was also receiving cyclophosphamide15 and another developed pulmonary thromboembolism in addition to deep vein thrombosis.16
In our patient, who had transient antiphospholipid antibodies, probably in relation to his leprosy, the combined use of thalidomide and prednisone may have acted as a trigger of the thrombotic event.
To conclude, we stress the importance of considering the risk of thromboembolic events in patients being treated with thalidomide for skin disorders, particularly when this drug is used in association with corticosteroids or chemotherapy agents or when other thrombophilic factors are present. More studies are needed to establish guidelines to prevent these serious complications in such patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Dr Carlos Zarco Olivo.
Please cite this article as: Hebe Petiti-Martin G, et al. Trombosis venosa profunda en paciente con lepra lepromatosa tratado con talidomida por leprorreacción. Actas Dermosifiliogr.2013;104:67-70.