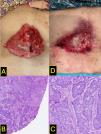
A 61-year-old woman with no significant past medical history presented in April 2022 with bleeding from a dorsolumbar lesion (Fig. 1A) that had been present for 10 years. Biopsy revealed an infiltrating basal cell carcinoma (BCC) (Fig. 1B–C). The patient was hospitalized due to severe, disabling low back pain. MRI of the thoracolumbar spine revealed the presence of a 11cm×2.8cm×5cm tumor at the level of the L1–L2 intervertebral space, with compression of the cauda equina roots and infiltration of the left paravertebral musculature and left psoas muscle.
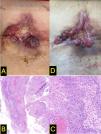
The case was staged as locally advanced BCC, stage IIIA (EADO classification), not amenable to surgery or radiotherapy. Treatment with vismodegib (150mg/day orally) was initiated in May 2022. An initial clinical improvement was observed (Fig. 1D, October 2022), which persisted until February 2023, when the patient experienced worsening pain and a newly developed mass at the lower right margin of the original lesion (Fig. 2A). Biopsy of the new lesion confirmed infiltrating cutaneous squamous cell carcinoma (SCC) (Fig. 2B–C). The case was reported to the Spanish Pharmacovigilance System, and pembrolizumab therapy was initiated. After 4 months into therapy, the patient showed clinical improvement (Fig. 2D), with reduced analgesic requirements and no relevant toxicity.
What is your diagnosis?
Diagnosis and commentsVismodegib is a hedgehog pathway inhibitor approved by the Spanish Agency for Medicines and Health Products (AEMPS) in August 2013 for the treatment of locally advanced and metastatic BCC.
Although the development of SCC within the tumor bed of a BCC is a rare finding, it has nonetheless been documented. In animal models, downregulation of hedgehog signaling predisposes to SCC formation.1 Furthermore, vismodegib may promote a shift toward squamous differentiation and keratinization in responsive patients.2 Several cases of SCC associated with vismodegib use have been reported, both in distant sites and within the tumor bed of the treated BCC.3 In this report, we describe a clinically significant case of SCC developing in the bed of a locally advanced BCC following initiation of vismodegib therapy. The initial biopsy only showed basal cell histologic features, without evidence of squamous differentiation (Fig. 1B). A case–control study demonstrated a >6-fold increased risk of developing non-BCC tumors within the same lesion in patients on vismodegib.4
Several hypotheses have been proposed for vismodegib-induced SCC, including selection of a preexisting SCC subpopulation; induction of squamous differentiation in malignant basal cells due to hedgehog pathway changes; and activation of the RAS/MAPK pathway due to SMO inhibition, which eliminates hedgehog pathway dependence for tumorigenesis. This process may allow surrounding epidermal cells, already carrying UV-induced mutational burdens, to undergo malignant transformation, with hedgehog pathway inhibition serving as the initiating event.4–6
The features of previously reported cases3,5,6 are shown in Table 1. Because of their observational nature and difficulty in quantifying UV- or radiation-induced field cancerization, a causal relationship cannot be definitively established. However, the temporal association between vismodegib initiation and subsequent SCC development suggests a possible link, warranting close dermatologic surveillance during therapy with vismodegib. To date, no SCCs have been reported in patients on sonidegib, another hedgehog pathway inhibitor approved for locally advanced BCC. Nonetheless, close monitoring is advisable for patients on this therapy and repeat biopsy should be considered for any lesion that fails to resolve or shows new proliferation.5 In some cases, local excision may be sufficient to treat the new SCC. In the present case, treatment was switched to an anti-PD-1 agent (pembrolizumab) due to concerns regarding the feasibility of complete surgical resection.
Conflict of interestThe authors declare that they have no conflict of interest.




 Artículo disponible en español
Artículo disponible en español