In recent years, the treatment of inflammatory disorders, including psoriasis, has been revolutionized by the introduction of new therapies, many of which have been developed using biotechnology (anti-tumor necrosis factor [TNF] agents and inhibitors of interleukin 12 and 23). However, not all patients respond favorably to biologic therapy: in some cases response to treatment is inadequate from the beginning (primary failure), in others it is initially good but subsequently wanes (secondary failure), and in some patients adverse events lead to withdrawal of the treatment.
When a patient does not respond to treatment with a biologic agent we can choose between a number of different strategies: increase the dose of the biologic agent or shorten the interval between treatments (intensify treatment), add a classic psoriasis treatment to the biologic regimen, switch the patient to another biologic agent in the same class (in the case of anti-TNF agents), or switch to treatment with a biologic with a different mechanism of action. Since the evidence currently available does not demonstrate which of these options is associated with the best outcome, the choice is empirical.
The reasons why biologic therapy fails are still poorly understood. One explanation for a lack of response may be that the mechanism involved in the patient's condition is not the mechanism blocked by the biologic agent used; this is the most likely cause for primary nonresponse. Another reason for failure may be the appearance of antibodies targeting the biologic agent, which neutralize its action or accelerate drug clearance. This phenomenon—called immunogenicity—presumably plays a greater role in secondary treatment failure.1 Any exogenous protein can induce a specific immune response leading to the formation of antibodies; in this way, a biologic agent can trigger antidrug antibodies (ADA) formation. The detection of ADA plays an important role in the assessment of the safety of biologic agents; they must be monitored in clinical trials, following the guidelines of the European Medicines Agency and the US Food and Drug Administration. However, such monitoring is not widespread in clinical settings and the practice is still somewhat controversial. An excellent article dealing with the implications of immunogenicity in biologic therapy was published recently in the Controversies in Dermatology section of Actas Dermo-Sifiliográficas.2
The production of ADAs depends on several factors. Some ADAs form as a result of the nature of the biologic agent: the size and structure of the protein, the type of protein (human or not), the presence of conjugates or fragments, and the conditions of formulation and storage. Other factors relate to the way treatment is administered (route, dose, duration of treatment, and dosing regimen). Patient factors (genetic profile, age, prior exposure, concomitant medication, and the condition being treated) should also be taken into account.
The assessment of immunogenicity is technically challenging because it is not easy to measure antibodies against antibodies.3 The techniques available have differing sensitivities and specificities and produce variable results.4,5 There is no standardized method for assessing immunogenicity: relevant ADA cutoffs and optimum drug levels in patients with psoriasis have yet to be established, and most of the techniques used do not distinguish between neutralizing and nonneutralizing ADAs. Moreover, immunogenicity is a dynamic process that evolves and changes over time. Consequently, transient antibody positivity, especially during the first few months of treatment, may have no real blocking effect on drug activity and can theoretically be managed by dose adjustment or the addition of immunomodulatory drugs to the regimen. Consequently, it is important to repeat the ADA measurement and to correlate the results obtained with clinical response. Enzyme-linked immunosorbent assay (ELISA) is currently the method most often used to measure ADA concentration because it is the simplest technique. An ELISA capture assay is used to measure the concentration of the biologic drug, and a bridge ELISA is used to measure levels of ADA. However, this procedure has a number of limitations: it does not detect immunoglobulin-G 4, ADAs are not detected in the presence of drug, and cross reactions with rheumatoid factor and/or activated complement can occur. The dose given and the timing of ADA measurement with respect to drug administration should always be taken into account in the interpretation of ADA levels.3 The only assay kits currently available on the market measure antibodies against infliximab, adalimumab, and etanercept.
In practice, what we want to know is whether the presence of ADAs is of clinical relevance. The first and most important repercussion of ADA production is the effect of the antibodies on the pharmacokinetics of the biologic agent. ADAs bind to the therapeutic protein and can neutralize its action and/or form immune complexes that lead to accelerated drug clearance, resulting in a loss of efficacy6-8 and reducing the survival (duration of continuous use) of the biologic treatment.9,10
Patients who develop ADA positivity also have a higher risk of adverse events7: infusion reactions in the case of infliximab,11 local reactions at the injection site in the case of golimumab,12 and thromboembolic events in patients with rheumatoid arthritis treated with adalimumab.13
Studies in rheumatology and gastroenterology have shown that the formation of ADA can be prevented or modulated by concomitant treatment with immunosuppressants, mainly methotrexate; however, no such studies have been undertaken in patients with psoriasis.4,5
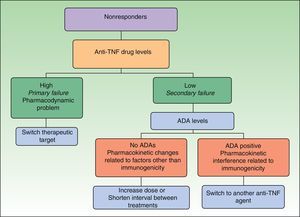
Immunogenicity studies can also provide useful information when we are choosing the most appropriate therapy for a patient who has not responded to initial treatment with an anti-TNF agent. Data concerning immunogenicity make it possible to develop decision algorithms based on drug levels, ADA levels, and clinical response.4,14,15 Such tools were recently presented in this journal,2 and here we include an algorithm validated by other authors16 that is applicable specifically to managing nonresponders (Fig. 1) because this is the situation where these tools are most useful for aiding clinical practice. With this algorithm, we introduce the concept of defining primary and secondary treatment failure on the basis of drug levels (as opposed to using clinical definitions based on the timing of nonresponse) and explain the different situations that can develop. In the case of primary failure, recommendations call for a switch to another biologic agent with a different mechanism of action. In the event of secondary failure, the recommended course of action is—in principle—to intensify the treatment regimen. However, the evidence shows that intensifying treatment does not result in renewed response in some patients and that as many as 10% of patients develop ADA against a TNF inhibitor during the first 4 weeks of treatment. When treatment failure is assessed on the basis of an objective measure—such as drug levels—primary failure is defined as a lack of clinical response in a patient with adequate drug levels and secondary failure refers to nonresponse when the serum drug level is low or undetectable. Neither the presence of ADAs or the timing of nonresponse are taken into account.17 The algorithm in Fig. 1 proposes a nonresponder be switched to an agent with a different mechanism of action (e.g., not a TNF inhibitor) if drug levels are high (primary failure), because such patients do not benefit from a higher dose of the TNF inhibitor or a switch to another drug in the same class. If the drug level is low (secondary failure), ADA levels should be assessed. If no ADAs are detected, the lack of response is the result of a pharmacokinetic problem unrelated to immunogenicity, such as an inadequate dose for the patient's weight, and the recommended course of action is a dose increase. However, if ADA levels are high, the abnormal pharmacokinetic profile is due to immunogenicity and the presumption is that the low drug levels are due to the immune response. Patients with high ADA levels should be switched to another biologic agent. The best choice would appear to be another anti-TNF agent because there are indications that patients who develop anti-TNF ADAs are those in whom this pathway is the most important, although this hypothesis not yet been demonstrated.14 What has been shown in patients receiving anti-TNF therapy is that nonresponders who are ADA positive have a better clinical response to a subsequent anti-TNF agent than nonresponders who are ADA negative.18 In patients with inflammatory bowel disease on infliximab, this switching strategy has been reported to cost an average of 30% to 50% less per patient than the dose escalation strategy, without compromising clinical response.19
Immunogenicity studies are also of some interest in patients whose response to treatment is acceptable, although not to the same degree. In responders, it is more difficult to standardize the timing of immunogenicity studies for each treatment, and interpreting the results is also more complicated. Once again, the first step should be to measure drug levels. If drug levels are high, the dose can be reduced with close monitoring of the clinical response. The problem is that since no therapeutic window (the range of optimum drug serum levels) has been defined in psoriasis, we lack a reference range to guide treatment. Furthermore, we already have skin disease activity markers to guide reductions in the intensity of dosing regimens for biologic therapy (decreasing the dose or increasing the interval between doses). The additional assessment of immunogenicity therefore seems less necessary. If drug levels are low, however, the ADA titer is measured, and if ADA levels are high, some authors recommend the drug be withdrawn because of the risk of adverse effects. We are also of the opinion that it is better to discontinue treatment in such cases because the ADAs are blocking the therapeutic agent and the patient's lack of symptoms may in fact be an indication of disease inactivity.
In summary, we believe that immunogenicity testing provides information that can be useful in guiding treatment decisions in patients receiving biologic therapy, particularly in nonresponders, switchers, and patients who have infusion reactions. Studies are still needed to determine the dose-response relationship for the biologic agents used to treat psoriasis and to identify the relevance of the presence of ADAs to clinical response. In any case immunogenicity studies should be seen as another tool for facilitating tailored management of the biologic dosing regimens and improving the cost-effectiveness of this therapy.
However, until now, immunogenicity in biologic therapy has only been studied in specialties such as rheumatology and gastroenterology and data for dermatology patients are scarce. We must therefore begin to produce data for our own patients in order to develop the tools that should underpin the management of biologic therapy in dermatology.
Please cite this article as: Rivera R, Herranz P, Vanaclocha F. Relevancia clínica de la inmunogenicidad en las terapias biológicas. Actas Dermosifiliogr. 2014;105:1–4.